
�Ȼ�ͭ��һ�ֹ㷺�����������ϡ���ľ�ķ������Ļ�����Ʒ��ij�о���ѧϰС���ô�ͭ��������Fe�������������Ʊ��Ȼ�ͭ���塣
��1������A��ϡ�����ܽ������ˮ�ܽ��ԭ����________��
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ�X��ѡ�������Լ��е�____������ţ���
| A��NaOH | B��NH3.H2O | C��CuO | D��Cu(OH)2E. CuSO4 |
��1������Cu2����Fe3�������ӷ���ˮ�ⷴӦ ��2��c d ��3������Ũ������ȴ�ᾧ
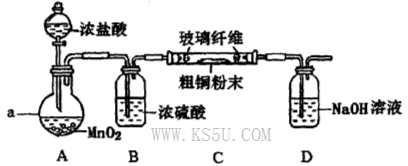
��4���� Բ����ƿ MnO2 + 4H++2Cl�� Mn2++ Cl2�� + 2H2O
Mn2++ Cl2�� + 2H2O
�� �� �� ��װ��C��D֮������һ��������װ��
���������������1����ͭ�к���Cu��Fe����������Ӧʱ��ʱCuCl2��FeCl3�����ڶ��߶���ǿ�������Σ�������ˮ�ⷴӦ����Cu(OH)2��Fe(OH)3��Ϊ������Cu2����Fe3�������ӷ���ˮ�ⷴӦͨ����ϡ�������ܽ⡣
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ��������������µ��������ӡ�������Ŀ���������ʣ���ѡ��CuO��Cu(OH)2��ѡ��ΪCD
��3������ȥFe(OH)3�����ĺ���CuCl2��Һ��һϵ�в����ɵ��Ȼ�ͭ���塣CuCl2��ǿ�������Σ�ˮ�����Cu(OH)2��HCl�������лӷ��ԣ����ӷ��ݳ����õ��Ĺ�����Cu(OH)2�����������ܽ�����¶ȵ�Ӱ��仯�ϴ����Կɲ��õIJ����ij�������Ϊ����Ũ������ȴ�ᾧ�����ˡ���Ȼ�����4��ʵ������ȡCl2����������ƿ����Ũ������������̹��ȵķ�����ȡ�ġ���Ӧ����������ʽΪ��MnO2 + 4H++2Cl�� Mn2++ Cl2�� + 2H2O������ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊû�б�Ҫ����ΪCu��HCl���ᷢ����Ӧ��ֻ��Cl2������Ӧ��������Cl2������NaOH������Ӧ�����µ������е�ѹǿ��С����ʱ�ձ��е���Һ������������װ�ã��ʸ�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ����װ��C��D֮������һ��������װ�á�
Mn2++ Cl2�� + 2H2O������ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊû�б�Ҫ����ΪCu��HCl���ᷢ����Ӧ��ֻ��Cl2������Ӧ��������Cl2������NaOH������Ӧ�����µ������е�ѹǿ��С����ʱ�ձ��е���Һ������������װ�ã��ʸ�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ����װ��C��D֮������һ��������װ�á�
���㣺����������Ʊ��������ķ��롢�ε�ˮ���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F�������ʵ���ɫ��Ӧ��Ϊ��ɫ�� A��B��C��D�����ᷴӦ������E������B�����ɡ��ֿ�ȼ�����壻��C��D�����ɡ�����ɫ��ζ������H����������ʹ�����ʯ��ˮ����ǡ�D��A�ɷ�Ӧ����C��F��HҲ�ɷ�Ӧ����C����������ɫ��ζ���塣
��ش��������⣺
��1��д��B��C�Ļ�ѧʽ��B��___________________; C��___________________;
��2��д��F��H2O��Ӧ�Ļ�ѧ����ʽ��__________________________________;
��3��д�����з�Ӧ�����ӷ���ʽ
��D��Һ+���_____________________________________________________;
��D��Һ+A��Һ��___________________________________________________;
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����A������KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
�ݴ˻ش��������⣺
��1��ͼ���漰������Һ������ķ�����__________________��
��2��B��C��D �������ʵĻ�ѧʽΪ��B_________ C_________ D_________
��3������E��ϡ���ᷴӦ�����ӷ���ʽΪ________________________________________��
��4��������F�д��ڵ����ֽ���Ԫ����ɵĺϽ�����100 mL 4mol/LHCl��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������m�����NaOH��Һ�����V�仯����ͼ��ʾ��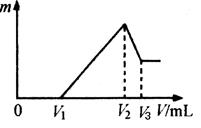
��֪V1��160mL������������Ϣ�ش�
��_________����ܡ����ܡ��������V3
��V2Ϊ_________mL����������������֣����ں�������д�������֣����������ں����������ȷ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϳ�����������ʢװ��Ũ���ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2������ȡ����6.0g����15.0mlŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y��
�ټ�ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+��Ӧѡ�� ��ѡ����ţ���
a��KSCN��Һ����ˮ b��NaOH��Һ
c��Ũ��ˮ d������KMnO4��Һ
����ͬѧȡ336ml����״��������Yͨ��������ˮ�У�������Ӧ��
SO2+Br2+2H2O=2HBr+H2SO4Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33g���ɴ���֪����Y��SO2���������Ϊ ��
��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��Q���塣Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�����ʡ�ԣ���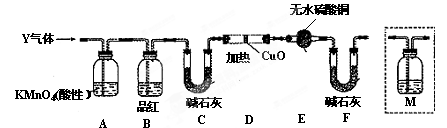
��3��װ��B���Լ��������� ��
��4����Ϊ����Y�л�����Q�������� ���û�ѧ����ʽ��ʾ����
��5��Ϊȷ��Q�Ĵ��ڣ�����װ��������M�� ��ѡ����ţ���
a��A֮ǰ b��A-B�� c��B-C�� d��C-D��
��6���������Y�к���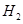 ��Ԥ��ʵ������Ӧ�� ��
��Ԥ��ʵ������Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C��DΪ�������ʣ�����B��C��D���³�ѹ��Ϊ���壬�ס��ҡ�������Ϊ������ҳ�����ΪҺ�壬������ɫ��ӦΪ��ɫ����ͼΪ��������֮������Ӧ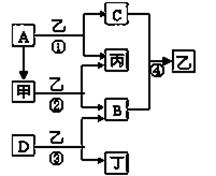
��1��д���������ʵĻ�ѧʽ��
A ��B ��D ���� ��
��2��������ʽΪ ����Ӧ��������11��2L����״���£�B���ɣ�����ת�Ƶĵ�
�ӵ����ʵ���Ϊ ��
��3��д����Ӧ�۵Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӻ�ͭ��Cu2S�������û���ͭ������ȡͭ���������·�Ӧ��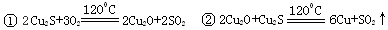
��������Cu2Sұ��ͭ����ȡCuSO4��5H2O������ͼ��
��Cu2S��ͭԪ�صĻ��ϼ�Ϊ ������1molCu2S��O2��Ӧ����2molCuʱ��ת�Ƶ��ӵ����ʵ����� ��
��Cu2O��CuO�м�������ϡ����õ�����ϵA�п�����Һ����ɫ�����к�ɫ�������ɣ���д�����ɺ�ɫ���ʵ����ӷ���ʽ ��
����ʹA�е����ܽ⣬�������м�����Լ������ ����ѡ����ĸ���ţ�
A��������HNO3 B��������NaOH C��������H2O2
�÷�Ӧ�����ӷ���ʽΪ ��
��ȡ5��00 g������Ʒ�������¶�ʹ��ֽ⣬�ֽ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��
����ͼ�п��Կ����������ֽ������¶��� ��
��ͨ������ȷ��258��ʱ������Ӧ�Ļ�ѧ����ʽΪ ��e���Ӧ�Ļ�ѧʽΪ �����������ȥ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���A12O3��SiO2������FeO·xFe2O3�������Ʊ�A12(SO4)3·18H2O����������������(���ֲ�����������):
��.�������м������ϡH2SO4������;
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ;
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
H2SO4�ܽ�A12O3�����ӷ���ʽ��
��KMnO4 ����Fe2+�����ӷ���ʽ���������� MnO4-+��Fe2++�� =
MnO4-+��Fe2++�� = Mn2++��Fe3+ +��
Mn2++��Fe3+ +��
��ʽ���������� ,���������� ��
��3����֪�������������������pH ks5u
| | Al��OH��3 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Fe2O3���й㷺����;����ͬѧ�Ķ��й����ϵ�֪���ڸ���������FeCO3���Եõ�Fe2O3��Ϊ�˽�һ����֤�˽��ۣ�����������ʵ�飺
| ʵ�鲽�� | ʵ����� |
| �� | ȡһ��������FeCO3�������������У������������������ټ��ᣬ��ȴ������ |
| �� | ȡ����ʵ�鲽��I���ù������һ�ྻ���Թ��У���������ϡ�����ܽ� |
| �� | ��ʵ�鲽���������Һ�еμ�KSCN��Һ����Һ��� |
 2Fe2O3��4CO2
2Fe2O3��4CO2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����(FeSO4��7H2O)�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ�� ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�;������ͨ���ij���壨��̬ʱ�������˹����꣩��д��������ĵ���ʽ ������Ϊ�Ϻ�����;���� ����ٻ�ڣ��������ǣ� ��
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���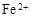 ��
��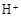 �⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
�⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ� �� ��
��5��;���ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ�� ����Ȼ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com