
����Ŀ������������ҵ����֮һ����ұ��ʯ��ҵ����ҩ�������ȷ��涼Ҫ�õ����ᡣ�����������Ƿ����ձ��У�������ˮ���ȣ��ټ�����Ũ���ᣬѸ�ٽ��裬�ų��������ȣ�ͬʱ�۲쵽������ڣ�������ͣ����ų��д̼�����ζ�����塣�Իش�
��1����������ˮ��ԭ����_________________________________________________��
��2�����ɵĺ�ɫ������______________��д����ѧʽ����
��3����������ͣ����ų��д̼�����ζ�����壨SO2������д����Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
��4����������������Ũ�����_________________������ţ�
������ ����ˮ�� ����ˮ�� ��ǿ������
��5����80mLŨ�����м���5.6gͭ������һ��ʱ��������ٷ�ӦΪֹ��ʵ���÷�Ӧ�й���1.12L����״���£�SO2�������ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________����Ӧ��ת�Ƶ���______mol��ͭʣ��_______g���÷�Ӧ��Ũ����������___________��__________��
���𰸡�Ũ���������ˮ���ʱ��ų��������ȣ��ӿ췴Ӧ C C+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O �ڢۢ� Cu+2H2SO4(Ũ)
CO2��+2SO2��+2H2O �ڢۢ� Cu+2H2SO4(Ũ) ![]() CuSO4+SO2��+2H2O 0.1 2.8 ǿ������ ����
CuSO4+SO2��+2H2O 0.1 2.8 ǿ������ ����
��������
�����������Ƿ����ձ��У�������ˮ���ȣ�����Ũ������ˮ��Ϸų��������ȣ��¶����ߣ��ӿ췴Ӧ���ʣ��ټ�����Ũ���ᣬѸ�ٽ��裬�ų��������ȣ�ͬʱ�۲쵽������ڣ�����Ũ�������ˮ�ԣ������DZ�Ϊ��̿�ڣ�̿�ں�Ũ�����ڼ��������·�����Ӧ���ɶ�������Ͷ�����̼���壬������ͣ����ų��д̼�����ζ�����塣
�ż�������ˮ��Ҫ������ˮ��Ũ�����Ϸų��������ȣ��ų�������ʹ�¶����ߣ��ӿ췴Ӧ���ʣ��ʴ�Ϊ��Ũ���������ˮ���ʱ��ų��������ȣ��ӿ췴Ӧ��
��Ũ���������ǻ�ϣ�Ũ���������ˮ�ԣ�ʹ���DZ�Ϊ̿�ڣ���˸����ɵĺ�ɫ������̼���ʴ�Ϊ��C��
�����ɵ�̼��Ũ�����������ȵ������·�Ӧ�ų�������̼�Ͷ����������壬��������ͣ����ų��д̼�����ζ�����壨SO2������д����Ӧ�Ļ�ѧ����ʽ��C+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O���ʴ�Ϊ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O���ʴ�Ϊ��C+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��
����Ũ���������ˮ�ԣ�Ũ������ˮϡ�Ͷ��ų��������ȣ�Ũ���������ˮ�ԣ���ʹ����̼������ڣ����ɵĺ�ɫ������C��̼��Ũ���ᷢ��������ԭ��Ӧ���ɶ�����̼�Ͷ����������壬���ж���������д̼�����ζ��Ũ����ǿ�����ԣ����������ˮ�ԡ���ˮ�ԡ�ǿ�����ԣ��ʢڢۢ���ȷ���ʴ�Ϊ���ڢۢܣ�
����80mLŨ�����м���5.6gͭ������һ��ʱ��������ٷ�ӦΪֹ��ʵ���÷�Ӧ�й���1.12L����״���£�SO2�������ɣ��÷�Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4(Ũ) ![]() CuSO4+SO2��+2H2O���ʵ�ΪCu+2H2SO4(Ũ)
CuSO4+SO2��+2H2O���ʵ�ΪCu+2H2SO4(Ũ) ![]() CuSO4+SO2��+2H2O����Ӧ������1.12L�����������弴0.05 mol����Ӧ��ת�Ƶ���2��0.05 mol =0.1 mol���ʴ�Ϊ0.1 mol��ͭ������0.05 mol��ͭʣ��5.6 ��0.05��64 = 2.4g���ʴ�Ϊ2.4 g��Ũ�������ɶ��������ϼ۽��ͣ�����ǿ�����ԣ�Ũ�����Ϊ����ͭ���������ԣ��ʴ�Ϊ��ǿ�����Ժ����ԣ�
CuSO4+SO2��+2H2O����Ӧ������1.12L�����������弴0.05 mol����Ӧ��ת�Ƶ���2��0.05 mol =0.1 mol���ʴ�Ϊ0.1 mol��ͭ������0.05 mol��ͭʣ��5.6 ��0.05��64 = 2.4g���ʴ�Ϊ2.4 g��Ũ�������ɶ��������ϼ۽��ͣ�����ǿ�����ԣ�Ũ�����Ϊ����ͭ���������ԣ��ʴ�Ϊ��ǿ�����Ժ����ԣ�


 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����Ȼ�ѧ��Ӧ����������ȷ����
A. HCl��NaOH��Ӧ���к��ȡ�H����57.3 kJ��mol1����H2SO4��Ca(OH)2��Ӧ���к��ȡ�H=2��(��57.3)kJ��mol1
B. ����ı�ȼ���Ȧ�H����890.3 kJ��mol1����CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H����890.3 kJ��mol1
C. ��֪��500�桢30MPa�£�N2(g)��3H2(g)![]() 2NH3(g) ��H����92.4kJ��mol��1����1.5 mol H2������N2�ڴ������³�ַ�Ӧ���ų�����46.2 kJ
2NH3(g) ��H����92.4kJ��mol��1����1.5 mol H2������N2�ڴ������³�ַ�Ӧ���ų�����46.2 kJ
D. CO(g)��ȼ������283.0kJ��mol1����2CO2(g) ===2CO(g)+O2(g)��Ӧ�ġ�H��+566.0 kJ��mol1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʽΪC9H18O2����������������ˮ�⣬���õ�����ʹ�����ͬ�����¶����������ܶ���ͬ�����ϴ�����������ͬ���칹��ĿΪ
A. 2 B. 8 C. 10 D. 16
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��H2��CH3OH���������Դ��
��1����֪���ֻ�ѧ�������������£�
��ѧ�� | C | O=O | C=O | C-O |
E/(kJ mol-1) | 958.5 | 497 | 745 | 351 |
2CO(g) +O2(g)==2CO2(g) ![]() H1 H2O(g)+CO(g)==H2(g) + CO2(g)
H1 H2O(g)+CO(g)==H2(g) + CO2(g) ![]() H2 = -41 kJmol-1
H2 = -41 kJmol-1
CH3OH(g)+ 3/2O2(g)==CO2(g)+2H2O(g) ![]() H3 = -660kJmol-1
H3 = -660kJmol-1
����H1=_____ kJmol-1����ӦCO(g)+2H2(g)![]() CH3OH(g)����H=_____ kJmol-1��
CH3OH(g)����H=_____ kJmol-1��
��2��һ�������£����ݻ�Ϊ2 L���ܱ�����Q�г���a mol CO��b molH2�ϳɼ״���CO(g) +2H2(g) ![]() CH3OH(g)�����ƽ��ʱ���������CH3OH������ٷֺ������¶ȡ� ѹǿ֮��Ĺ�ϵ��ͼ1��ʾ��ͼ2��ʾ��һ���¶��£�H2��ƽ��ת�����뷴Ӧ��ʼʱ���ַ�Ӧ���Ͷ�����ʵ���֮�ȣ���X��ʾ����ѹǿ֮��Ĺ�ϵ��
CH3OH(g)�����ƽ��ʱ���������CH3OH������ٷֺ������¶ȡ� ѹǿ֮��Ĺ�ϵ��ͼ1��ʾ��ͼ2��ʾ��һ���¶��£�H2��ƽ��ת�����뷴Ӧ��ʼʱ���ַ�Ӧ���Ͷ�����ʵ���֮�ȣ���X��ʾ����ѹǿ֮��Ĺ�ϵ��
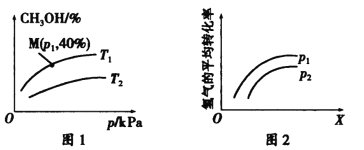
��ѹǿ��ͬʱ���¶�ΪT1��T2ʱ����Ӧ�ﵽƽ������Ҫ��ʱ��ֱ�Ϊt1��t2�������֮�����Դ�СΪt1___ t2(����>������<������=��������ȷ����)��
��P1_____P2(����>������<������=��������ȷ����)��
����a =2��b=4����ѹǿΪP1���¶�ΪT1ʱ�÷�Ӧ��ƽ�ⳣ��K=______________��
������ѹǿΪP1���¶�ΪT1ʱ����Q������ͬʱ��������ʵ�����CO��H2��CH3OH�������壬��Ӧ��ʼʱ��v(CH3OH)��_____v(CH3OH)��(����>������<������=��������ȷ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ʵ�����Na2CO3��mH2O��BaCl2��nH2O�Ļ����3.68g����������ˮ���裬����ַ�Ӧ��ɵ�1.97g��������m��nֵ�ֱ���
A.10��2B.7��3C.3��1D.1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������0.1mol/L��NaHCO3��Һ250mL��������в��裺
��1���õ�����ƽ��ȡNaHCO3����______________g��
��2�����ƺõ�NaHCO3�������______________��������������ˮ����___________��
��3������Һ_____________����Һ����______________����250mL����ƿ�У��ڲ��������в�����ʧ�κ�һ��Һ�壬�����ʹ��Һ��Ũ��ƫ_________����ͣ���
��4������������ˮ��ϴ_______________2-3�Σ���ϴ��Һ����______________�У�
��5��������ƿ�ڼ�ˮ������̶���______________ʱ������________________С�ļ�ˮ��___________________������ˮ�����̶��ߣ��������ҺŨ��ƫ_________����ͣ���Ӧ��_____________��
��6�����Ǻ�ƿ�ǣ�___________��Ȼ����õ���Һ�����Լ�ƿ�У����ñ�ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ϊ375%��Ũ�����ܶ�Ϊ116 g/cm3����250mLŨ��Ϊ1mol/L��ϡ���ᡣ�Իش��������⣺
��1������ϡ����ʱ��Ӧѡ������Ϊ______mL������ƿ��
��2����������Ҫ______ mLŨ���ᣬ����ȡʱ��ѡ��������Ͳ�е�______��������ĸ��
A. 5 mL B. 10 mL C. 25 mL D. 50 mL
��3������ȡŨ������������в�����
�ٵ�ϡ�ͺ�������¶�������һ�º��ز�����������ע������ƿ�С�
��������ƿ��С�ļ�����ˮ��Һ��ӽ����α���2��3 cm�������ý�ͷ�ιܼ�����ˮ��ʹ��Һ�İ���ײ���ƿ���Ļ��α������С�
����ʢ������ձ���ע������ˮ��ʮ���������ò�����������ʹ���Ͼ��ȡ�
��������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һȫ��ע������ƿ��
���������У���ȷ��˳���ǣ�����ţ�________________________��
��4�����������ƹ����У��øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᣬ�����Ƶ�ϡ����Ũ����__________���ƫ�ߡ�����ƫ�͡�������Ӱ�족������δ������ˮϴ���ձ��ڱڻ�δ��ϴ��Һע������ƿ�������Ƶ�ϡ����Ũ����_______���ƫ�ߡ�����ƫ�͡�������Ӱ�족����
��5���������Ӧ������ƿ�е�ϡ����ת�Ƶ�_______�д�ţ������ϱ�ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪CO2�����Һ������Ӧʱ�����η����������ӷ�Ӧ��CO2��2OH����CO32����H2O��CO2��CO32����H2O��2HCO
��1����0.01mol�������Ƶ�ʯ��ˮ��ͨ��CO2���õ�0.4g��ɫ��������ͨ��CO2�����ʵ���Ϊ______mol��______mol��
��2������NaOH��Ba(OH)2�����Һ100mL����֪����Һ��c(OH��)=1mol/L������Һ�л���ͨ��CO2���壨��Һ����仯���Բ��ƣ���
�ٵ�����ͨ��CO2�������Ϊ0.56L����״̬��ʱ���ɵij�����࣬��ԭ��Һ���������ƺ��������������ʵ���Ũ�ȸ�Ϊ���٣�__________________
�ڵ�ͨ���CO2���������Ϊ2.24L����״̬��ʱ����Һ�������ӣ�OH�����⣩�����ʵ���Ũ���Ƕ��٣�______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֳ��������ᣬΪ��֤��������������ʣ�ijͬѧ��չ����Ϊ��������������ʵ�ʵ���о�����̽�����
��1����������о�������CH3COOH���о�Ŀ����_____���о�������ʵ���о���
��2����ͬѧ��������·�����
A.������һ������0.10mol/L CH3COOH��Һ��Ȼ�����Һ��pH����pH����1�����֤������Ϊ������ʡ�
B.������0.01mol/L��0.10mol/L��CH3COOH���ֱ�����ǵ�pH�������ߵ�pH���С��1����λ�����֤��������������ʡ�
C.�Ȳ�0.10mol/LCH3COOH��pH��Ȼ���������100�棨�����Ǵ���ӷ������ٲ�pH�����pH��С�����֤��������������ʡ�
D.����һ������CH3COONa��Һ������pH����������pH����7�����֤��������������ʡ�
����Ϊ�����������е���____������ѡ��
��3����ش������������⣺
��������ʵ��ص���____������ѡ��
A.��ˮ��Һ��ȫ������ B.��ˮ��Һ�в��ֵ���
C.ˮ��Һ������ D.ˮ��Һ�д��ڵ���ƽ��
��д������ĵ��뷽��ʽ��____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com