
���н�������A��B��C�������ҡ���������D��E��F��G��H������֮�䷢�����·�Ӧ��ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û��ȫ���������

�����������Ϣ�ش��������⣺
��1������F�Ļ�ѧʽΪ ��
��2��˵������ɫ�����ҵ�һ����; ��
��3������E��F������Һ��ķ����� ��ʵ���Ҽ���G�������Ľ�������ʱ������G����Һ�м��� ��Һ��
��4����Ӧ�ٵ����ӷ���ʽΪ ��
��5������F��H��ת���辭�������������еķ�Ӧ����д����������Ӧ�Ļ�ѧ����ʽ�� �� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ۡ��ߺ����ö���ȼ��֮һΪLiH����֪���з�Ӧ��
��2Li(s)��H2(g)===2LiH(s)��H1����182 kJ��mol��1��
��2H2(g)��O2(g)===2H2O(l)��H2����572 kJ��mol��1��
��4Li(s)��O2(g)===2Li2O(s)��H3����1 196 kJ��mol��1��
��LiH��O2��ȼ�յ��Ȼ�ѧ����ʽΪ
A��2LiH(s)��O2(g)===Li2O(s)��H2O(l)��H����702 kJ��mol��1
B��2LiH(s)��O2(g)===Li2O(s)��H2O(l)��H����1 950 kJ��mol��1
C��2LiH(s)��O2(g)===Li2O(s)��H2O(l)��H����1 586 kJ��mol��1
D��2LiH(s)��O2(g)===Li2O(s)��H2O(g)��H����988 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�������£���2 L�ܱ������г���3 mol X(g)��1 mol Y(g)���з�Ӧ��2X(g)��Y(g)  3Z(g)��2W(g)��ijʱ�̲ⶨ�����Ũ�ȣ������ܵ��ǣ� ��
3Z(g)��2W(g)��ijʱ�̲ⶨ�����Ũ�ȣ������ܵ��ǣ� ��
A��Z��0��75 mol / L B��Z��1 mol / L  C ��W��1 mol / L D��W��0��8 mol / L
C ��W��1 mol / L D��W��0��8 mol / L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꺣��ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
����ɫ��ѧ�����У�����״̬�Ƿ�Ӧ���е�ԭ��ȫ��ת��Ϊ���ƵõIJ������CH3C��CH�ϳ�CH2=C(CH3)COOCH3����ʹԭ�������ʴﵽ��ߣ�����Ҫ�ķ�Ӧ����( )
A��H2��CO2 B��CO2��H2O C��CO��CH3OH D��CH3OH��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꺣��ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���н���ʹ�õ�ⷨұ����õ���
A ���� B��п C��ͭ D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���Ĵ�ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
����ϩ���Ҵ�������ɵĻ�������У���̼Ԫ�ص������ٷֺ���Ϊ60%������Ԫ�ص������ٷֺ���Ϊ
A��15.6% B��26.7% C��30% D����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���Ĵ�ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��������˳����ȷ����
A. ԭ�Ӱ뾶��O��N��C
B. ���ȶ��ԣ�H2O��HF��H2S
C. ���ԣ�KOH��NaOH��Mg(OH)2
D. ���ԣ�H3PO4��H2SO4��HClO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������һ�и߶���ѧ����ĩ���ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л���X�Ľṹ��ʽ����ͼ��ʾ���������й�˵���в���ȷ����
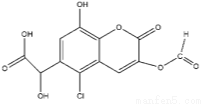
A���ܷ����ӳɡ�ȡ����������Ӧ
B��1 mol������������7mol NaOH��Ӧ
C��1 mol������������6 mol H2��Ӧ
D������FeCl3��Һ������ɫ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
ʵ�����б仯���л���Ӧ�����ͣ�����ȷ����( ��
A��CH3CH3��CH3CH2Clȡ����Ӧ
B��CH2�TC H2��CH2BrCH2Br�ӳɷ�Ӧ
H2��CH2BrCH2Br�ӳɷ�Ӧ
C��CH2�TCH2��CH3CH2OHȡ����Ӧ
D��CH��CH��CHBr�TCHBr�ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com