
·ÖĪö ¢ŁAŗĶEČÜŅŗĻŌ¼īŠŌ£¬ĪŖ¼ī»ņĒæ¼īČõĖįŃĪ£®øł¾ŻĄė×Ó¹²“ęæÉÖŖ¶žÕßĪŖBa£ØOH£©2”¢Na2CO3£¬ÓÉÓŚ0.1mol/LµÄAČÜŅŗµÄpHŠ”ÓŚ13£¬ĖłŅŌAŹĒNa2CO3£»EŹĒBa£ØOH£©2£»
¢ŚŌŚBČÜŅŗÖŠÖš½„¼ÓČė°±Ė®ÓŠ°×É«³ĮµķÉś³É£¬¼ĢŠų¼ÓČė°±Ė®ÖĮ¹żĮ棬³ĮµķĻūŹ§£¬ŌņBČÜŅŗÖŠŗ¬ÓŠAg+£¬¹ŹŅõĄė×ÓĪŖNO3-£¬ĖłŅŌBĪŖAgNO3ČÜŅŗ£»
¢ŪŌŚCČÜŅŗÖŠ¼ÓČėĢś·Ū£¬ČÜŅŗµÄÖŹĮæŌö¼Ó£¬ĖµĆ÷CČÜŅŗŗ¬ÓŠFe3+£¬ÓÉÓŚČÜŅŗÖŠĄė×Ó²»ÖŲø“³öĻÖ£¬ĖłŅŌDČÜŅŗÖŠŗ¬ÓŠAl3+£»
¢ÜŌŚDČÜŅŗÖŠ¼ÓČė¹żĮæBa£ØOH£©2ČÜŅŗ£¬Ć»ÓŠ³Įµķ£¬ĖµĆ÷DČÜŅŗ²»ŗ¬ÓŠSO42-£¬ĖłŅŌŅõĄė×ÓĪŖCl-£¬SO42-ŌŚCČÜŅŗÖŠ£¬ĖłŅŌCŹĒFe2£ØSO4£©3£»DŹĒAlCl3£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗ¢ŁAŗĶEČÜŅŗĻŌ¼īŠŌ£¬ĪŖ¼ī»ņĒæ¼īČõĖįŃĪ£®øł¾ŻĄė×Ó¹²“ęæÉÖŖ¶žÕßĪŖBa£ØOH£©2”¢Na2CO3£¬ÓÉÓŚ0.1mol/LµÄAČÜŅŗµÄpHŠ”ÓŚ13£¬ĖłŅŌAŹĒNa2CO3£»EŹĒBa£ØOH£©2£»
¢ŚŌŚBČÜŅŗÖŠÖš½„¼ÓČė°±Ė®ÓŠ°×É«³ĮµķÉś³É£¬¼ĢŠų¼ÓČė°±Ė®ÖĮ¹żĮ棬³ĮµķĻūŹ§£¬ŌņBČÜŅŗÖŠŗ¬ÓŠAg+£¬¹ŹŅõĄė×ÓĪŖNO3-£¬ĖłŅŌBĪŖAgNO3ČÜŅŗ£»
¢ŪŌŚCČÜŅŗÖŠ¼ÓČėĢś·Ū£¬ČÜŅŗµÄÖŹĮæŌö¼Ó£¬ĖµĆ÷CČÜŅŗŗ¬ÓŠFe3+£¬ÓÉÓŚČÜŅŗÖŠĄė×Ó²»ÖŲø“³öĻÖ£¬ĖłŅŌDČÜŅŗÖŠŗ¬ÓŠAl3+£»
¢ÜŌŚDČÜŅŗÖŠ¼ÓČė¹żĮæBa£ØOH£©2ČÜŅŗ£¬Ć»ÓŠ³Įµķ£¬ĖµĆ÷DČÜŅŗ²»ŗ¬ÓŠSO42-£¬ĖłŅŌŅõĄė×ÓĪŖCl-£¬SO42-ŌŚCČÜŅŗÖŠ£¬ĖłŅŌCŹĒFe2£ØSO4£©3£»DŹĒAlCl3£¬
£Ø1£©ÓÉŅŌÉĻ·ÖĪöæÉÖŖ£¬AĪŖNa2CO3£¬BĪŖAgNO3£¬CĪŖFe2£ØSO4£©3£¬EĪŖBa£ØOH£©2£¬¹Ź“š°øĪŖ£ŗNa2CO3£»AgNO3£»Fe2£ØSO4£©3£»Ba£ØOH£©2£»
£Ø2£©AĪŖNa2CO3£¬ĪŖĒæ¼īČõĖįŃĪ£¬Ė®½ā³Ź¼īŠŌ£¬Ė®½āĄė×Ó·½³ĢŹ½ĪŖCO32-+H2O?HCO3-+OH-£¬
CŹĒFe2£ØSO4£©3ČÜŅŗ£¬¼ÓČė×ćĮæµÄĢś·Ūŗ󣬷“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2Fe3++Fe=3Fe2+£¬
¹Ź“š°øĪŖ£ŗCO32-+H2O?HCO3-+OH-£» 2Fe3++FeØT3 Fe2+£®
µćĘĄ ±¾ĢāŅŃĄė×Ó¹²“ęŗĶĄė×Ó·“Ó¦ĪŖŌŲĢåæ¼²éĪŽ»śĪļµÄĶʶĻ£¬²ąÖŲѧɜµÄ·ÖĪöÄÜĮ¦µÄ漲飬ŅŌ“Ė¶ŌĄė×ÓĶʶĻ£¬ŹĒøßæ¼µÄČȵćŗĶÄŃµć£®ŌŚ½ā“ĖĄąĢāŹ±£¬Ź×ĻČ½«ĢāÖŠÓŠĢŲÕ÷µÄĪļÖŹĶĘ³ö£¬Č»ŗó½įŗĻĶĘ³öµÄĪļÖŹĶʵ¼Ź£ÓąµÄĪļÖŹ£¬×īŗó½ųŠŠŃéÖ¤¼“æÉ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 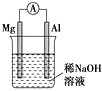 Ķ¼ĀĮʬ·¢ÉśµÄµē¼«·“Ó¦Ź½ŹĒ£ŗAl+4OH-3e-ØTAlO2-+2H2O | |
| B£® |  Ķ¼·¢ÉśĪöĒāøÆŹ“£¬Ąė×Ó·“Ó¦·½³ĢŹ½ĪŖ£ŗFe+2H+ØTFe2++H2”ü | |
| C£® | 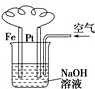 Ķ¼ČÜŅŗÖŠ·¢ÉśĮĖ±ä»Æ£ŗ4Fe£ØOH£©2+O2+2H2OØT4Fe£ØOH£©3 | |
| D£® | 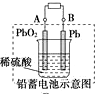 Ķ¼³äµēŹ±£¬Ńō¼«·“Ó¦ŹĒ£ŗPbSO4+2H2O-2e-ØT=PbO2+SO42-+4H+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĶĘ¹ćŹ¹ÓĆŗ¬Į×Ļ“µÓ¼Į | B£® | ÓĆ¹¤ŅµĪŪĖ®Ö±½Ó¹ąøČÅ©Ģļ | ||
| C£® | ÓĆO3Ģę“śCl2×÷ŅūÓĆĖ®Ļū¶¾¼Į | D£® | ŗ¬Hg2+µÄ·ĻĖ®Ö±½ÓÅÅ·Å |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
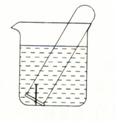 Ķ¼ĖłŹ¾Ė®²ŪÖŠµÄŹŌ¹ÜÄŚÓŠŅ»Ć¶Ģś¶¤£Øŗ¬ÉŁĮæµÄĢ棩£¬·ÅÖĆŹżĢģŗó¹Ū²ģ£ŗ
Ķ¼ĖłŹ¾Ė®²ŪÖŠµÄŹŌ¹ÜÄŚÓŠŅ»Ć¶Ģś¶¤£Øŗ¬ÉŁĮæµÄĢ棩£¬·ÅÖĆŹżĢģŗó¹Ū²ģ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SO2µÄĖ®ČÜŅŗæÉŅŌµ¼µēĖµĆ÷ŹĒSO2µē½āÖŹ | |
| B£® | ¼ÓČČNaOHČÜŅŗŹ±²»ÄÜŹ¹ÓĆ²£Į§ŹŌ¹Ü£¬ŅņĪŖ²£Į§ÖŠµÄSiO2ÄÜÓėNaOH·“Ó¦ | |
| C£® | ijĪŽÉ«ČÜŅŗÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ²¢¼ÓČČ£¬²śÉśµÄĘųĢåÄÜŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬øĆČÜŅŗŅ»¶ØÓŠNH4+ | |
| D£® | ijČÜŅŗÖŠ¼ÓČėŃĪĖįÄܲśÉśŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢ壬ŌņøĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCO32-»ņHCO3- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
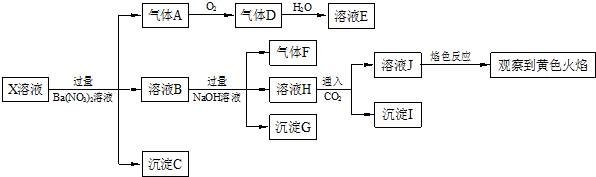
| A£® | XÖŠæĻ¶Ø“ęŌŚNa+”¢Fe2+”¢A13+”¢NH4+”¢SO42- | |
| B£® | ĘųĢåF¾“ß»ÆŃõ»ÆæÉÖ±½ÓÉś³ÉĘųĢåD | |
| C£® | ³ĮµķCŅ»¶ØŹĒBaSO4”¢³ĮµķGŅ»¶ØŹĒFe£ØOH£©3”¢³ĮµķIŅ»¶ØŹĒAl£ØOH £©3 | |
| D£® | XÖŠ²»ÄÜČ·¶ØµÄĄė×ÓŹĒ A13+”¢Na+”¢K+ŗĶC1- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģ¼ĖįøĘÓė“×ĖįČÜŅŗ·“Ó¦£ŗCaCO3+2H +ØTCa2++CO2”ü+H2O | |
| B£® | ³ĪĒåŹÆ»ŅĖ®ŗĶŃĪĖį·“Ó¦£ŗOH-+H+ØTH2O | |
| C£® | ÓĆNa2O2ČÜÓŚĖ®£ŗNa2O2+H2OØT2Na++2OH-+O2”ü | |
| D£® | Ę«ĀĮĖįÄĘČÜŅŗĶØČė¹żĮæCO2£ŗAlO2-+4CO2+2 H2OØTAl3++4 HCO3- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com