


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢ� | B���ڢܢ� | C���٢ۢ� | D���ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ�����Ʊ���������һ�����ܱ�������ͨ����н��� |
| B�����ף�P4����Na��Ӧ������ˮ�� |
| C�����ӽ���Ƥ���ϻ�����ʹ�У�������NaOH��Һ��ϴ |
| D�����Ҵ������Ỻ�����뵽Ũ�����н�����ȣ��ټ�����ȡ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
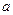 mol��L
mol��L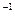 NaOH��Һ (2)
NaOH��Һ (2)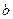 mol��L
mol��L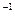 Na2CO3��Һ (3)c mol��L
Na2CO3��Һ (3)c mol��L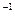 HCl��Һ
HCl��Һ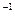 AgNO3��Һ (5)��̪��Һ (6)������Һ
AgNO3��Һ (5)��̪��Һ (6)������Һ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ԭ����ͭ��ʵ��ʱ��ͨ�������ž�װ���еĿ����ټ��� |
| B��ϡ��Ũ����ʱ����Ũ�������ջ�������ע��ˮ�в����Ͻ��� |
| C�����Թ��е�Һ�����ʱ���Թܿڲ��ܶ����Լ������� |
| D���÷���м����������ʱ���Ƚ�����м���ڼ�Һ�м���1��2min |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������������ƿ���ܽⲢϡ�����̶ȣ����ó�һ�����ʵ���Ũ�ȵ���Һ |
| B���ò�����պȡ��Һ������ʪ���pH��ָ�ϲⶨ��pH |
| C����NaOH��Һϴ�Ӳ����ղ�˿���ٽ�����ɫ��Ӧ |
| D����ȡ�ζ�����Һ�����������Ӷ������¶���ƫС |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���þƾ��Ƶ������������ |
| B�����Թ����Һ�壬�Թܿڲ��ܶ��� |
| C����ȡ50������Һ����10��������Ͳ��5�� |
| D�������и�ʴ��ҩƷ��ֱ�ӷ���������ƽ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ʊ����ھƾ��� |
| B������ᱣ���ڲ����Լ�ƿ�� |
| C������������Һ����ڴ����������Լ�ƿ�� |
| D������������������������ʢװŨ�����Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������� | B���������� | C��Ư�� | D��̼���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com