
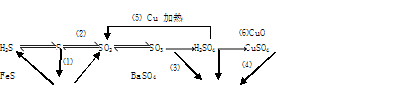
 CuSO4+SO2��+2H2O�� (2��)
CuSO4+SO2��+2H2O�� (2��)


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��S�Ļ�ԭ�� | B��S��Ư���� | C��SO2�Ļ�ԭ�� | D��SO2��Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
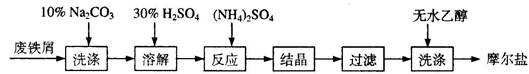
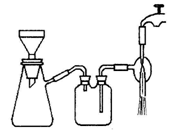
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ƻ��� | B����Ư�� | C����ϴ | D��ҽҩ����ըҩ��ũҩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ɫ����ζ | B���ж� | C����ʹƷ����ɫ | D���ǽ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 6CaSiO3��P4O10 10C��P4O10
6CaSiO3��P4O10 10C��P4O10 P4��10CO
P4��10CO C6H6O6��2H����2I�� 2
C6H6O6��2H����2I�� 2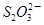 ��I2
��I2
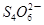 ��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��
��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��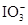 ��5
��5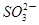 ��2H��
��2H�� I2��5
I2��5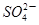 ��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ��
��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ�� 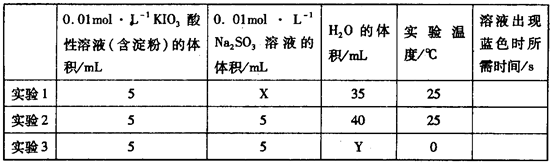
 Ce3����
Ce3���� H2O2��
H2O2�� H2O
H2O
 Ce��OH��4����
Ce��OH��4���� ______________
______________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��Ӧ�������� ��Ӧ��
��Ӧ�������� ��Ӧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com