
ij����С���������ʵ��װ�ã���֤��������̼��ˮ�Ӵ�ʱ���ܺ������Ʒ�Ӧ����
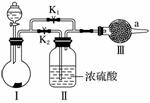
(1)���������������̼��Ӧ�Ļ�ѧ����ʽ��____________________________________���÷�Ӧ�У���Ӧ���������__________(����ڡ���С�ڡ�)���������������
(2)װ�â��е��Լ���__________(����ĸ)
a��ϡ���� b��ϡ����
c��С�մ� d��ʯ��ʯ
(3)ʵ��ʱ��Ӧ�ȴ��ɼ�__________(�K1����K2��)���۲쵽Ԥ������ر������ٴ���һ�����ɼС�
(4)ʵ������н������ǵ�ľ������a�ڣ��۲쵽�����ǵ�ľ��ʼ�ղ���ȼ��
��Ϊ�۲쵽����ľ����ȼ�������ڢ��װһ��ʢ�м�ʯ�ҵĸ���ܣ�Ŀ����________________________________________________________________________��
������Ϊ��ʹ���ɼĽ��������չ۲쵽ľ����ȼ��Ҳ����֤��CO2�����˷�Ӧ��ԭ����________________________________________________________________________
________________________________________________________________________��
(5)Ϊ��һ���ﵽʵ��Ŀ�ģ�Ӧ�����ʵ���ǣ�ȡ���շ�Ӧ��������ù��壬________________________________________________________________________��
�𰸡�(1)2Na2O2��2CO2===2Na2CO3��O2������
(2)bc��(3)K2
(4)�ٳ�ȥδ��ַ�Ӧ��CO2
��ˮ��Na2O2��Ӧ��������
(5)����ϡ���ᣬ������������ͨ�����ʯ��ˮ��(�𰸺�������)
������(1)�˷�Ӧ�Ƿ��ȷ�Ӧ��(2)����ʵ��Ŀ�ģ���Ҫ�����CO2������װ�â�����ȡCO2װ�ã���ֻ��ѡ��С�մ���Ϊ�����ӷ���HCl������Ӧѡ�����ᡣ(3)��ͨ����CO2��Ȼ����ͨʪ��CO2���жԱȣ�(5)֤����Ӧ������к���CO ��
��


 �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����Ȼ�ѧ����ʽ��
��H2(g)��1/2O2(g)===H2O(g)����H1��a kJ·mol��1
��2H2(g)��O2(g)===2H2O(g)����H2��b kJ·mol��1
��H2(g)��1/2O2(g)===H2O(l)����H3��c kJ·mol��1
��2H2(g)��O2(g)===2H2O(l)����H4��d kJ·mol��1
���й�ϵʽ����ȷ����(����)
A��a<c<0���������������� B��b>d>0
C��2a��b<0 D��2c��d>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NaHCO3��NaOH�Ļ��������ܱ������м��ȣ����й��ڻ�������ǰ��������������ʵ������ж���ȷ����(����)
A������ǰ���ĵö�
B�����Ⱥ����ĵö�
C������ǰ��һ����
D����NaOH����ʱ�Ż�һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���Ӧ�У������ﲻ�淴Ӧ������Ӧ��������仯���仯����(����)
A��Na��O2 B��NaOH��CO2
C��NaHCO3��NaOH D��Na2CO3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ʹ�Ա�ڷɴ��еõ�һ���ȶ��ġ����õ����滷����һ���ڷɴ��ڰ�װʢ��Na2O2��K2O2������װ�ã�������;�Dz������������й���Na2O2��������ȷ����(����)
A��Na2O2�����������ӵĸ�����Ϊ1��1
B��Na2O2�ֱ���ˮ��CO2��Ӧ������ͬ����O2ʱ����Ҫˮ��CO2���������
C��Na2O2�ֱ���ˮ��CO2��Ӧ������ͬ����O2ʱ��ת�Ƶ��ӵ����ʵ������
D��Na2O2��Ư��ԭ����SO2��Ư��ԭ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ijԭ���ڴ����������״̬ʱ����Χ�����Ų�ʽΪ4d15s2��������˵����ȷ����(����)
A����Ԫ��ԭ�Ӵ����������״̬ʱ��ԭ���й���3��δ�ɶԵ���
B����Ԫ��ԭ�Ӻ����5�����Ӳ�
C����Ԫ��ԭ�ӵ�M�ܲ㹲��8������
D����Ԫ��ԭ������㹲��3������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��DΪ������Ԫ�أ�A��Bͬ���ڣ�C��Dͬ���壬��֪A����������D�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��B�������Ӻ�C�������ӵ��Ӳ�ṹ��ͬ����C���ӵĺ˵��������B���ӵģ���4��Ԫ�صĵ縺��˳����ȷ����(����)
A��A>B>C>D B��D>C>B>A
C��C>D>B>A D��A>B>D>C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾΪ�ӹ��������з���X�����ַ���������ݷ���1�ͷ���2ָ������˵���к�������(����)
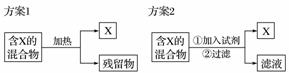
A������ѡ�÷���1����̼�������к��е��Ȼ��
B������1�еIJ�����Ӧ�þ��е������������ӷ�
C������2�м�����Լ�һ���ܹ����X������ʷ�����ѧ��Ӧ
D������2�м���NaOH��Һ���Է����SiO2��Fe2O3������е�Fe2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H��O�����γ�H2O��H2O2���ֻ�����Ը����й���Ϣ����������⣺
(1)ˮ��ά��������������һ�����ʡ�
��1 mol������________mol�����
�������ģ�ͱ�ʾ��ˮ���ӽṹ��____________��


(2)��֪H2O2���ӵĽṹ��ͼ��ʾ��H2O2���Ӳ���ֱ���εģ�������ԭ�������ڰ�չ������������ϣ�������ԭ�����鼹λ���ϣ���ҳ�н�Ϊ93��52�䣬������O—H����O—O���ļнǾ�Ϊ96��52�䡣
�Իش�
��H2O2���ӵĵ���ʽ��______________���ṹʽ��________________________________________________________________________��
��H2O2�����Ǻ���________����________����__________(����ԡ��Ǽ��ԡ�)���ӡ�
��H2O2������CS2����Ҫ˵�����ɣ�________________________________________________________________________
________________________________________________________________________��
��H2O2����Ԫ�صĻ��ϼ���__________����Ҫ˵��ԭ��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com