
A��B��C��D������ѧ��ѧ���������ʣ�����A��B��C������ͬһ��Ԫ�ء���һ�����������ת����ϵ����ͼ��ʾ����ش��������⣺

��1����A��B��C����Һ���Լ��ԣ�CΪ���Ƹ��ķ��ͷ۵���Ҫ�ɷ�֮һ��
��A��������ѧ������Ϊ___________��D�ĵ���ʽΪ___________��
��25��ʱ��0.1mol•L-1 B��C��Һ��pH�ϴ����___________��Һ(�����ʵĻ�ѧʽ)��д��B��Һ���Ե����Ե�ԭ��___________����֪��B��Һ���pH=10������ˮ���������H+��Ũ��Ϊ________________��
��2����Ӧ��ϵ��ͬʱͨ����顢������ˮ��������������Ҫ��ѧ��Ӧ�У�
I��CH4(g)+2O2(g)�TCO2(g)+2H2O(g)��H1=-802.6kJ/mol
II��CH4(g)+O2(g)�TCO2(g)+2H2(g)��H2=-322.0kJ/mol
III��CH4(g)+H2O(g)�TCO(g)+3H2(g)��H3=+206.2kJ/mol
����CH4(g)+2H2O(g)�TCO2(g)+4H2(g)��H4=+165.0kJ/mol
��ش��������⣺
��CH4��ȼ���ȡ�H________ ��H1��(���������������=��)��
���ڷ�Ӧ��ʼ�Σ���ӦII�ķ�Ӧ���ʴ��ڷ�ӦIII�ķ�Ӧ���ʣ��ȽϷ�ӦII�Ļ��EII�ͷ�ӦIII�Ļ��EIII�Ĵ�С��EII ________EIII(���������������=��)��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���㽭ʡͩ���и߶������У���ͨ�ࣩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��װ���ܴﵽʵ��Ŀ�ĵ���
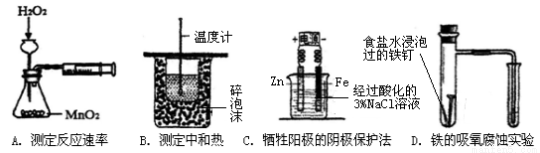
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶���ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���ʵ���Ũ����ͬ��������NaX��NaY��NaZ����Һ����pH����Ϊ8��9��10����HX��HY��HZ��������ǿ������˳����( )��
A��HX��HZ��HY B��HX��HY��HZ C��HZ��HY��HX D��HY��HZ��HX
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016��������������ѧԺ������ѧ�߶�����ĩ��ѧ���������棩 ���ͣ�ʵ����
��ͼ����0.1000 mol/L������ζ�ijδ֪Ũ�ȵ�NaOH��Һ��ʾ��ͼ��ij�εζ�ǰ�����ʢ������ζ�����Һ���λ�á���ش�
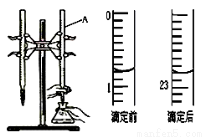
��1�� ����A�������� ��
��2�� ���������������ζ�ǰ����Ϊ mL���ζ������Ϊ mL ��
��3�� ijʵ��С��ͬѧ������ʵ���ʵ���������±���ʾ�� ���ݱ������ݼ�����Ĵ���NaOH��Һ��ƽ��Ũ���� mol/L��(������λ��Ч����)
ʵ�� ��� | ����NaOH��Һ�������mL�� | �ζ�ǰ����� ���������mL�� | �ζ�������� ���������mL�� |
1 | 20.00 | 1.20 | 23.22 |
2 | 20.00 | 2.21 | 24.21 |
3 | 20.00 | 1.50 | 23.48 |
��4�����÷�̪��ָʾ�����жϵ���ζ��յ��ʵ��������_____________
��5�� �����м��ּٶ�����������ۣ�(���Ӱ�족����ƫ�ߡ�����ƫ�͡�)
�� ���ζ�ǰ��ƿδ�ô���Һ��ϴ���Բⶨ�����Ӱ���� ��
��ȡ����Һ�ĵζ��ܣ��ζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ��_________
�۱�Һ����ʱ�����ζ�ǰ���ӣ��ζ����ӣ��Բⶨ�����Ӱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016��������������ѧԺ������ѧ�߶�����ĩ��ѧ���������棩 ���ͣ�ѡ����
250 ���1.01��105 Paʱ����Ӧ2N2O5(g) == 4NO2(g)��O2(g) ��H����56.76 kJ/mol���Է����е�ԭ���ǣ� ��
A�������ȷ�Ӧ B���Ƿ��ȷ�Ӧ
C�����ؼ��ٵķ�Ӧ D��������ЧӦ��������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶�����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ȱ����ͬ���칹��(�����ǿռ��칹)��ĿΪ
A��3�� B��4�� C��5�� D��6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����������·һ�и�һ��12���¿���ѧ���������棩 ���ͣ�ѡ����
�����й����ữѧ���ʵ������У���ȷ���� ( )
A�����ڷ��õ�Ũ����Ũ�ȱ�С����ΪŨ�������ˮ��
B���������Na2SO3��Ӧ�Ƶ�SO2����
C��Ũ������ֽ�ų���NO2���ܽ���������ʻ�ɫ
D����������Fe2O3��Ӧ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵ��Ĵ��¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
��O2��HClת��ΪCl2�������Ч�棬������Ⱦ��
��1����ͳ�ϸ�ת��ͨ������ͼ��ʾ�Ĵ���ѭ��ʵ�֣����У���Ӧ��Ϊ��2HCl(g) + CuO(s)  H2O(g)+CuCl2(g) ��H1����Ӧ������1molCl2(g)�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ , (��Ӧ���á�H1�͡�H2��ʾ)��
H2O(g)+CuCl2(g) ��H1����Ӧ������1molCl2(g)�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ , (��Ӧ���á�H1�͡�H2��ʾ)��
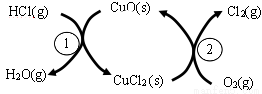
��2������RuO2����������HClת��ΪCl2���ܷ�Ӧ���и��õĴ����ԡ�
��ʵ������һ��ѹǿ�£��ܷ�Ӧ��HClƽ��ת�������¶ȱ仯��aHCl��T������ͼ12�����ܷ�Ӧ�ġ�H 0 ,�����������������������A��B�����ƽ�ⳣ��K(A)��K(B)�нϴ���� ��
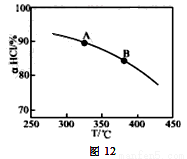
��������ʵ������ѹ�����ʹѹǿ��������ӦaHCl��T��HCl��ת�������¶ȵĹ�ϵ�����ߵ�ʾ��ͼ������Ҫ˵�����ɣ� ��
�����д�ʩ�����������aHCl���� ��
A������n(HCl) B������n(O2) C��ʹ�ø��õĴ��� D����ȥH2O
��3��һ�������²�÷�Ӧ������n(Cl2)���������£�
t(min) | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n(Cl2)/10-3mol | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
2.0��6.0min����HCl�����ʵ����仯��ʾ�ķ�Ӧ���� ����mol��min-1Ϊ��λ����
��4��Cl2��;�㷺��д����Cl2�Ʊ�Ư�۵Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ��һ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ba(OH)2��Һ����������Һ�У�ʹSO42-ȫ��ת����BaSO4��������ʱ��Ԫ�ص���Ҫ������ʽ�ǣ� ��
A��Al3+ B��Al(OH)3 C��AlO2- D��Al3+��Al(OH)3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com