
��8�֣�Ϊ��֤������Cl2 > Fe3�� > SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��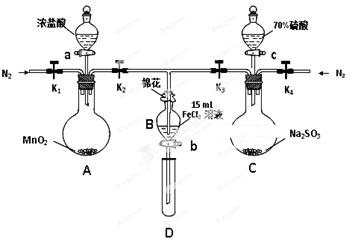
ʵ����̣�
I. ���ɼ�K1~K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��. ����a���μ�һ������Ũ���ᣬ��A���ȡ�
��. ��B����Һ���ʱ��ֹͣ���ȣ��ر�K2��
��. ����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��. ��K3�ͻ���c������70%�����ᣬһ��ʱ���ر�K3��
��. �����Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1�����̢��Ŀ���� ��
��2�����н������ҺΪ ��
��3��A�з�����Ӧ�Ļ�ѧ����ʽ ��
��4��������ȡ��SO2ͨ�����Ը��������Һ��ʹ��Һ��ɫ�������ӷ���ʽΪ ��
��5���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬�������±���ʾ�����ǵļ����һ���ܹ�֤��������Cl2 > Fe3�� > SO2���� ����ס����ҡ�����������
| | ���̢�B��Һ�к��е����� | ���̢�B��Һ�к��е����� |
| �� | ��Fe3����Fe2�� | ��SO42�� |
| �� | ����Fe3������Fe2�� | ��SO42�� |
| �� | ��Fe3����Fe2�� | ��Fe2�� |
��1���ų�װ���еĿ�������ֹ���� ��1�֣� ��2��NaOH��Һ ��1�֣�
��3��MnO2��4HCl(Ũ MnCl2��Cl2����2H2O ��2�֣�
MnCl2��Cl2����2H2O ��2�֣�
��4��2MnO4����5SO2��2H2O��2Mn2����5SO42����4H�� ��2�֣���5���ҡ��� ��2�֣�
���������������1�����̢��Ŀ����ʹװ�ó����������ų�װ���еĿ�������ֹ���ţ���2��Ũ������MnO2������Ӧ����������������HCl���Ǵ�����Ⱦ�Ϊ�˷�ֹ��Ⱦ������Ӧ���������Ƕ��ܷ�����Ӧ�ļ�����Һ���������н������ҺΪNaOH��Һ����3����A�з�����Ӧ��ȡ�����Ļ�ѧ����ʽ��MnO2��4HCl(Ũ MnCl2��Cl2����2H2O����4����C��������Na2SO3������Ӧ����SO2, SO2�л�ԭ�ԣ����KMnO4��ԭΪMn2+��������ȡ��SO2ͨ�����Ը��������Һ��ʹ��Һ��ɫ�����ݵ����غ�ɵ������ӷ���ʽΪ2MnO4����5SO2��2H2O��2Mn2����5SO42����4H������5����B����FeCl2��Һ��������ͨ��Cl2����������Ӧ��2FeCl2+Cl2="2" FeCl3������Һ�к���Fe3+����Fe3��> SO2������B��ͨ��SO2ʱ�ᷢ����Ӧ2Fe3��+ SO2+2H2O=2Fe2++SO42-+4H+;����Һ��Ӧ�ú���SO42-���ڹ��̢� B��Һ�к��е�SO42-���ӣ�����ֻҪ���̢� B��Һ�к��е�Fe3�����ӣ��ڹ��̢� B��Һ�к��е�SO42-���ӣ��Ϳ���֤�������ԣ�Cl2 > Fe3�� > SO2������ʵ�����ҡ�����
MnCl2��Cl2����2H2O����4����C��������Na2SO3������Ӧ����SO2, SO2�л�ԭ�ԣ����KMnO4��ԭΪMn2+��������ȡ��SO2ͨ�����Ը��������Һ��ʹ��Һ��ɫ�����ݵ����غ�ɵ������ӷ���ʽΪ2MnO4����5SO2��2H2O��2Mn2����5SO42����4H������5����B����FeCl2��Һ��������ͨ��Cl2����������Ӧ��2FeCl2+Cl2="2" FeCl3������Һ�к���Fe3+����Fe3��> SO2������B��ͨ��SO2ʱ�ᷢ����Ӧ2Fe3��+ SO2+2H2O=2Fe2++SO42-+4H+;����Һ��Ӧ�ú���SO42-���ڹ��̢� B��Һ�к��е�SO42-���ӣ�����ֻҪ���̢� B��Һ�к��е�Fe3�����ӣ��ڹ��̢� B��Һ�к��е�SO42-���ӣ��Ϳ���֤�������ԣ�Cl2 > Fe3�� > SO2������ʵ�����ҡ�����
���㣺����Cl2 �� Fe3���� SO2������ǿ���Ƚϵ���֤������������֪ʶ��


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��21�֣�ʵ����������80mL��1.5 mol/L��NaHCO3��Һ���Իش�
��1����ʵ�����ʹ�õIJ������� ��
��2�����ø���ҺʱӦ��ȡNaHCO3������Ϊ_____________________����3�����в���������������Һ���ʵ���Ũ�ȵ�Ӱ�죨��д��Ӱ�졢ƫ��ƫ�ͣ�
A�����ƹ�����δϴ���ձ��Ͳ�������
B������ƿʹ��֮ǰδ��ɣ�����������ˮ��
C������ʱ��������ƿ�Ŀ̶��ߣ�
D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦����__________
д�����з�Ӧ�����ӷ���ʽ��
A����NaHCO3��Һ�еμ�����
B����Ba(OH)2��Һ�еμ�����NaHCO3��Һ
C����ˮ�еμ�MgCl2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
8��)��18��4mol��L-1�ܶ�Ϊ1��84g��mL-1��Ũ���ᣬ����100mLŨ��Ϊ1mol��L-1��ϡ���ᣬ������������£�
| A������Ͳ��ȡһ�������Ũ���ᣬ����ע��װ��Լ50mL����ˮ���ձ�����ò��������衣 |
| B����Լ30 mL����ˮ���ֳ�����ϴ���ձ��Ͳ���������ÿ��ϴ��Һ��ע������ƿ� |
| C����ϡ�ͺ������С�ĵ��ò���������������ƿ�� |
| D������������ƿ�м�����ˮ��Һ�����̶�����1cm��2cm�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ÿ��1�֣���8�֣�ʵ������Ҫ����0.1 mol/L CuSO4��Һ480 mL�������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������_______ _��________�Լ�����������Ƭ��ֽ��
��2�����㣬Ӧѡ��������ȷ________
| A����ҪCuSO4����8.0g | B����ҪCuSO4��5H2O����12.0 g |
| C����ҪCuSO4��5H2O����12.5 g | D����ҪCuSO4����7.7 g |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣�ijʵ��С����MnO2��Ũ�����ϼ�����ȡCl2�������Ƶõ������볱ʪ��Ca(OH)2���巴Ӧ��ȡ����Ư�ۡ�ʵ��װ������ͼ��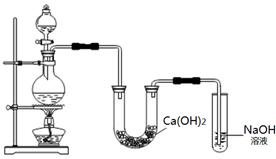
�ش��������⡣
��1��Ư�۵���Ч�ɷ��� ��д��ѧʽ����
��2����ƿ�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3���¶Ƚϸ�ʱ��������ʯ�ҷ�����Ӧ��6Cl2 + 6Ca(OH)2��5CaCl2 + Ca(ClO3)2 + 6H2O���÷�Ӧ���������� ����ԭ���� ������Ӧ����0.3mol Cl2��ת�Ƶĵ�����Ϊ mol��
��4������ѧ��ѧ�У��������������������� �� ��д���֣�����������ԭ���������� ��
��д���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��������������Ӧ�ö�Ӧ��ϵ��ȷ����
| A���ƺͼصĺϽ���е����ԣ������ڿ����ӷ�Ӧ���Ƚ����� |
| B����������ˮ�ܲ���Al(OH)3���壬��������ˮ�� |
| C����ȩ��ʹ�����ʱ��ԣ�������ʳƷ������ |
| D��ˮ����������ˮ�������������ϼ��ͷ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ʵ���У�Ҫ��ʹ�Ȼ�����Һ�е�Al3+��ȫ���������������ѡ�������Լ��е�
A��ʯ��ˮ B������������Һ c������ D����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й������仯�����˵���в���ȷ����
| A����ҵ���ڸ�������CO��ԭ��Fe2O3������ʯ���� |
| B�����ڴ�����ȼ�ջ�����º�ˮ������Ӧ���ܵõ�Fe3O4 |
| C����ҵ�Ͽ��������������桢����Ũ���ᡢŨ���� |
| D���Ȼ�����Һ�н�ǿ�����ԣ��ʿ�������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��5�֣������£�����A��B��C�ֱ�Ϊ���塢����ɫ��������ɫ���壬�ں��������£����ǿ�����������̽��з�Ӧ����֪E��Һ����ɫ�ġ���ش�
��1��д��E���ʵĻ�ѧʽ_______________��
��2��д��G��H�Ļ�ѧ����ʽ_____________________________________________��
��3��д��B��F��D�����ӷ���ʽ___________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com