
�������仯�����ڹ�ũҵ���������������ж�������Ҫ��Ӧ�á�
(1)Ca(ClO)2��NaClO��NaClO2�Ⱥ��Ȼ����ﶼ�dz��õ���������Ư��,����Ϊ���Ƕ�����������������,��д����ҵ����������NaOH��Һ��Ӧ����������NaClO�����ӷ���ʽ��������������������������������
(2)�ػ�ɫǿ�̼�������Cl2OΪ���ʹ��ϸ�Ч��ȫ���������֮һ,ʵ���ҿ��ó�ʪ��Cl2��Na2CO3��Ӧ��ȡ����Cl2O��NaHCO3�����ӷ���ʽ������������������������������������
(3)��ɫ����ClO2��������ˮɱ��������ˮ������
��KClO3��SO2��ǿ������Һ�з�Ӧ���Ƶ�ClO2,�˷�Ӧ�����ӷ���ʽΪ����������������������������
��ClO2��Ũ���������Cl2,ÿ����1 mol Cl2ת�Ƶ��ӵ����ʵ���Ϊ����������
��ClO2�ɽ���ˮ�е�Mn2+ת��ΪMnO2����ȥ,������ԭΪCl-,�÷�Ӧ�������������뻹ԭ�������ʵ���֮��Ϊ����������
(4)��Cl2����ijЩ�����л���ʱ�����������HCl�����÷�Ӧ4HCl+O2 2Cl2+2H2O,��ʵ���ȵ�ѭ�����á�
2Cl2+2H2O,��ʵ���ȵ�ѭ�����á�
��֪:��������Ӧ��,4 mol HCl�������ų�115.6 kJ��������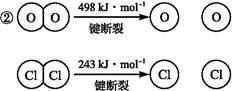
��Ͽ�1 mol H��O����Ͽ�1 mol H��Cl�������������ԼΪ�������� kJ,H2O��H��O����HCl��H��Cl��(�ǿ��������)����������
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A��B��C��D��E��F���ֳ����������֪���ǰ�������������K����Ag����Na����
Ba2����Fe2����Al3������������Cl����OH����AlO2-��NO3-��SO42-��CO32-�������Ƿֱ����0.1 mol/L����Һ��������ʵ�飺
�ٲ����ҺA��C��E���ʼ��ԣ��Ҽ���A��E��C��E����ɫ��dz��ɫ������ɫ�ܲ����۲죩������B��Һ�еμ�ϡ��ˮ�������������ɳ����������ȫ���ܽ⣻����F��Һ�еμ�ϡ���ᣬ��Һ����ػ�ɫ��������ɫ�������ɣ�����D��Һ�еμ�Ba��NO3��2��Һ����������
��1��д��A��D��E��F�Ļ�ѧʽ��
A________��D________��E________��F________��
��2�������ӷ���ʽ����C��Һ�ʼ��Ե�ԭ��__________________________________��
��3��д��ʵ����з�Ӧ�����ӷ���ʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni��OH��2��̿�ۡ���������Ϳ�����������Ƴɣ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о�������Ƴ����ʵ������ͼ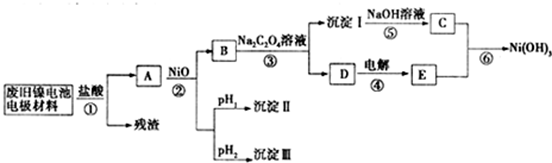
��֪����NiCl2������ˮ��Fe3+��������Ni2+
��ij�¶���һЩ�������������Ksp����������������pH�����ʾ��
| M��OH��n | Ksp | pH | |
| ��ʼ���� | ������ȫ | ||
| Al��OH��3 | 1.9��10-33 | 3.43 | 4.19 |
| Fe��OH��3 | 3.8��10-38 | 2.53 | 2.94 |
| Ni��OH��2 | 1.6��10-14 | 7.60 | 9.75 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B��C��D��E�����ǵ������ӿ�����Na+�� ��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-��
��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-�� ����֪��
����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
�ش��������⣺
��1���������У�һ��û�е���������_________________________________________________��
��������������ͬ�������εĻ�ѧʽ��_____________________________________________��
��2��D�Ļ�ѧʽΪ_________��D��Һ�Լ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ��________________��
��3��A��C����Һ��Ӧ�����ӷ���ʽ��______________________________________________��
E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��______________________________________________________��
��4����Ҫ����B�������������ӣ���ȷ��ʵ�鷽����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����֣�ʳ���еĿ�����������軯�أ��仯ѧʽΪK4[Fe(CN)6]��3H2O��42.2 g K4[Fe(CN)6]��3H2O��Ʒ������ˮ���̵���������(��Ʒ�������¶ȵı仯����)����ͼ��ʾ��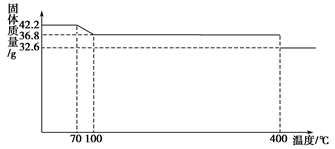
�Իش��������⣺
(1)��ȷ��150 ��ʱ�������ʵĻ�ѧʽ_____________��
(2)��������֪����Ȼ�����軯���������Ժܵͣ�����ˮ��Һ���ᷴӦ�ų��������軯��(HCN)���壻�����軯�ؼ�����һ���¶�ʱ�ֽܷ�����軯��(KCN)���ݴ��жϣ����ʳƷʱӦע�������Ϊ_____________________________________________________��
(3)��Fe2����Fe3���Ĵ������£���ʵ��2SO2��O2��2H2O��2H2SO4��ת������֪����SO2�ķ���ͨ�뺬Fe2����Fe3������Һ��ʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2����O2��4H����4Fe3����2H2O������һ����Ӧ�����ӷ���ʽΪ__________________________��
(4)��֪Fe(OH)3���ܶȻ�����Ksp��1.1��10��36������ʱ��FeCl3��Һ�еμ�NaOH��Һ������ҺpHΪ3ʱ��ͨ������˵��Fe3���Ƿ������ȫ_____________��(��ʾ����ij����Ũ��С��10��5 mol��L��1ʱ������Ϊ�����ӳ�����ȫ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�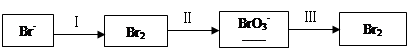
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ��� �������ƹ��壻�� ͭ˿���� �Ȼ������壻�� ϡ����� ������̼���壻 ������������Һ���� ̼���Ʒ�ĩ���� ���Ǿ��壻�� �����Ȼ��ƣ����������գ�
��1������״̬�¿ɵ������________����2�����ڵ���ʵ���________����3�����ڷǵ���ʵ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ű�Ҫ��д�����з�Ӧ�����ӷ���ʽ
��������ͭ��������
��̼������Һ�к�������������Һ���
��2�������������ʣ���NaCl���� ��Һ̬HCl ��CaCO3���� ������KCl �����Ǣ�ͭ��CO2��H2SO4��KOH���� ��ˮ�������CH3COOH �Ѿƾ�������ţ�
�������������ܵ������_________________________________��
���������������ڵ���ʵ���_____________________________��
���������������ڷǵ���ʵ���___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������͵�������(NOx)�Դ�����Ⱦ�������أ��о�����������Ⱦ�ķ����ǻ�ѧ�����ߵ���Ҫ���⣬Ŀǰ�кܶ��ַ�������������Ⱦ��
��1�������ü������ԭNOx�ķ�������NOx����Ӧ���£�
CH4(g)+4NO2(g)�� 4NO(g)+CO2(g)+2H2O(g)����H�� ��574 kJ��mol��1
CH4(g)+4NO(g)�� 2N2(g)+CO2(g)+2H2O(g)����H�� ��1160 kJ��mol��1
��CH4(g)+2NO2(g)�� N2(g)+CO2(g)+2H2O(g)����H�� ��
��2����������β���ķ���֮һ�����������ϰ�װ��ת�������������·�Ӧ��2NO(g)+2CO(g) N2(g)+2CO2(g)����H��0��
N2(g)+2CO2(g)����H��0��
����һ���¶��£���2molNO��1molCO����1L�̶��ݻ��������У�15���Ӻ�ﵽƽ�⣬��Ӧ�����и����ʵ�Ũ�ȱ仯��ͼ����ʾ����ƽ�ⳣ��K= (С�������3λ)��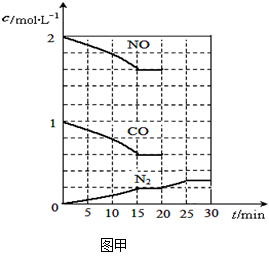
���������¶Ȳ��䣬20minʱ���������г���CO����0.6mol��ƽ�⽫ �ƶ�(����������ҡ�����)��
���������¶Ȳ��䣬20minʱ��ԭƽ�������г���CO��N2��0.6mol��ƽ�⽫ �ƶ�(����������ҡ�����)��
��20minʱ�����ı䷴Ӧ����������N2Ũ�ȷ�����ͼ��ʾ�ı仯����ı������������
(����ĸ)��
| A��������� | B�������¶� | C����С������� | D������CO2���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com