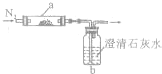
��2011?������ģ��ȡ��ѧʽΪXY�ĺ�ɫ��ĩ״�������������ʵ�飮��XY���������Ƹ����̼�۳�ֻ�ϣ�ƽ���ڷ�Ӧ��a�У���bƿ��ʢ��������ʯ��ˮ������ͼ������������ʵ�飮
ʵ�鿪ʼʱ����ͨ�뵪������һ��ʱ����ȷ�Ӧ��a����Ӧ�����Ż��Ƶġ����ȡ�����ͬʱ��bƿ����Һ���ְ�ɫ���ǣ�����Ӧ��ȫ��ֹͣ���ȣ��Լ���ͨ�뵪����ֱ����Ӧ����ȴ����ʱ���������Ϻ�ɫ�������ɣ�
��������ʵ��ش��������⣺
��1��Ԫ��y��
��
��
����Ԫ�����ƣ���
��2��ֹͣ����ǰ�Ƿ���Ҫ�ȶϿ�a��b�����ӣ�Ϊʲô��
����Ҫ����Ϊ�е�������ͨ�룬bƿ��Һ���ᵹ����a��
����Ҫ����Ϊ�е�������ͨ�룬bƿ��Һ���ᵹ����a��
��
��3����Ӧ��a�з��������з�Ӧ�Ļ�ѧ����ʽ��
CuO+C
CO+Cu
2CuO+C
CO
2+2Cu
C+CO
22CO
CuO+CO
Cu+CO
2CuO+C
CO+Cu
2CuO+C
CO
2+2Cu
C+CO
22CO
CuO+CO
Cu+CO
2��
��4����ʵ���β���Ƿ���Ҫ����������Ҫ��������ش���δ������粻��Ҫ��������˵�����ɣ�
�账�����������йش���ƿ�����ϴ������ȼ������������й�װ������ͭ��ĩ�ķ�Ӧ��
�账�����������йش���ƿ�����ϴ������ȼ������������й�װ������ͭ��ĩ�ķ�Ӧ��
��
��5��Ԫ��X�ĵ������ܽ���ϡ���ᣬ������Ӧ�����ӷ���ʽΪ
3Cu+4H++2NO3-=Cu2++2NO+4H2O
3Cu+4H++2NO3-=Cu2++2NO+4H2O
��

 ��2011?������ģ��ȡ��ѧʽΪXY�ĺ�ɫ��ĩ״�������������ʵ�飮��XY���������Ƹ����̼�۳�ֻ�ϣ�ƽ���ڷ�Ӧ��a�У���bƿ��ʢ��������ʯ��ˮ������ͼ������������ʵ�飮
��2011?������ģ��ȡ��ѧʽΪXY�ĺ�ɫ��ĩ״�������������ʵ�飮��XY���������Ƹ����̼�۳�ֻ�ϣ�ƽ���ڷ�Ӧ��a�У���bƿ��ʢ��������ʯ��ˮ������ͼ������������ʵ�飮






 +��n-1��H2O
+��n-1��H2O
 +��n-1��H2O
+��n-1��H2O ��
�� ��
��
 ��
�� ��
��

