
 ������2Ŀ�ģ� ��
������2Ŀ�ģ� ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | ʵ�鲽�� | Ԥ������ͽ��� |
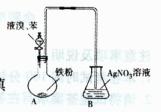
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���__________ | ��������ų��ҳ���ʯ��ˮδ�����ǣ������________������ |
| �� | | |
| �� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��[(b��a)/84]��22.4��1000 mL |
| B��[(b��c)/31]��22.4��1000 mL |
| C��[(c��a)/106]��22.4��1000 mL |
| D��[(c��a)/106]��2��22.4��1000 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
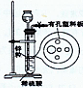
| A���٢ۢ� | B���٢ڢ� | C���ܢݢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
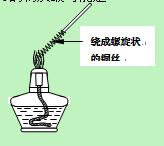
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� | FeSO4 | H2SO4 | Ag2SO4 | Al2(SO4)2 | ���� |
| ���������������� | 15.0 | 7.0 | 0.40 | 0.34 | 5.0 |
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 |
| FeSO4��Һ�ȣ�g�� | 15.6 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 |
| Al2(SO4)3�ܽ�ȣ�g�� | 31.2 | 33.5 | 36.4 | 40.4 | 45.7 | 52.2 |
�鿴�𰸺ͽ���>>
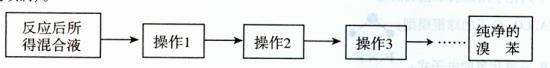
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
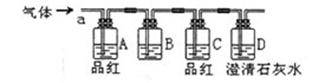
| A��NaCl��Һ | B��KMnO4��Һ | C������ | D������ʯ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2NH3(g)+CO2(g)
2NH3(g)+CO2(g)| �¶�/�� | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 |
| ƽ����ѹǿ/kPa | 5.7 | 8.3 | 12.0 | 17.1 | 24.0 |
| ƽ��������Ũ��/mol?L-1 | 2.4��10-3 | 3.4��10-3 | 4.8��10-3 | 6.8��10-3 | 9.4��10-3 |
| A��2v(NH2)=v(CO2) | B���ܱ���������ѹǿ���� |
| C���ܱ������л��������ܶȲ��� | D���ܱ������а���������������� |
 NH4HCO2+NH3?H2O
NH4HCO2+NH3?H2O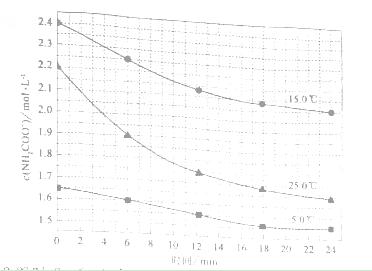
 ʱ��0-6min ���������ˮ�ⷴӦ��ƽ������ ______��
ʱ��0-6min ���������ˮ�ⷴӦ��ƽ������ ______���鿴�𰸺ͽ���>>
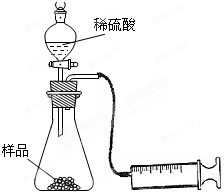
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3COOH+Cu2O��+2H2O����������ɣ�����������̽����
CH3COOH+Cu2O��+2H2O����������ɣ�����������̽����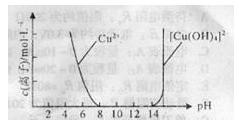
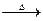 CH3COOH+Cu2O��+2H2O�����ɵ����ɣ�
CH3COOH+Cu2O��+2H2O�����ɵ����ɣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com