



 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ���ݰ�У��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��8�֣���ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǹ��壬B��G��Һ�壬�����Ϊ���壬F�Ǻ���ɫ���塣������ͼ��ϵ�ƶϣ�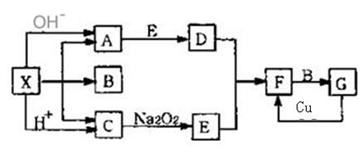
(1)��ѧʽ��X ___________��
(2) д��C����E�Ļ�ѧ��Ӧ����ʽ______________________________________
(3) ��д��F��B��Ӧ����G�Ļ�ѧ����ʽ��____________________���÷�Ӧ��������������ͻ�ԭ��������ʵ���֮��Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
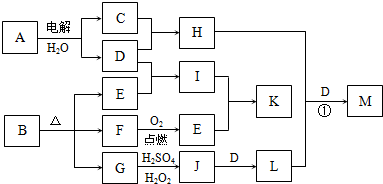
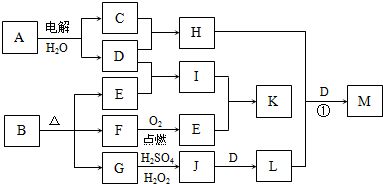
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�ɶ�����Ԫ����ɵ�һЩ����֮���ת����ϵ��ijЩ��Ӧ��������ȥ�����������ʾ�йص�һ�ַ�Ӧ��������ijЩ�����Ѿ���ȥ��������A��B��D�ڳ����¾�Ϊ��ɫ�̼�����ζ�����壬C��ʹʪ��ĺ�ɫʯ����ֽ���������壬M���������ɫҺ�塣
�� ����F�Ļ�ѧʽ__________
�� ����B�ĵ���ʽ__________
�� д��C��E�Ļ�ѧ����ʽ______________________________
�� д��G��E�����ӷ���ʽ______________________________

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com