
| A��ʵ���ҿ�ͨ������ķ������Ӻ�Fe3+������ˮ�л����������ˮ |
| B������Ʒ�м��������ữ���Ȼ�����Һ����ȷ����Ʒ���Ƿ���SO42- |
| C���ýྻ�IJ�����պȡ����Һ��Ʒ��Һ���ھƾ��ƻ��������գ�����Ƿ���Na+ |
| D���ø����ҽྻ�IJ�����պȡ������Һ����ʪ���pH��ֽ�в���������ɫ�����տɲⶨ��Һ��pHֵ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������A��B��֧�ྻ�Թ��У����� | ���������� |
| ����2����A�Թ��м��������Ba��OH��2��Һ�����ã����� | �а�ɫ���� |
| ����3��ȡ��������2�õ������������������ | ����������ȫ�ܽ⣬˵�������к������� |
| ����4��ȡ��������2�õ�����Һ�� | |
| ����5����B�Թ��м��� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | 2Na+2H2O=2NaOH+H2�� 3NO2+H20=NO+2HNO3 | ��Ϊˮ����������������ԭ��Ӧ |
| B | SiO2����NaOH��Һ��ӦҲ��������ᷴӦ Al2O3����NaOH��Һ��Ӧ��Ҳ�������ᷴӦ | �����������Ϊ���������� |
| C | Cl2+2Br-=2Cl-+Br2 Zn+Cu2+=Zn2++Cu | ��Ϊ���ʱ���ԭ���û���Ӧ |
| D | Cl2+2FeCl2=2FeCl3 I2+SO2+2H2O=H2SO4+2HI | �������ӷ�Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
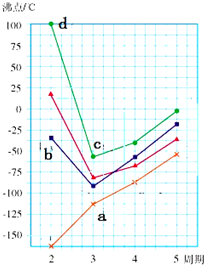 A��B��D��E��F��G��ԭ������������������ֶ�����Ԫ�أ�A��B���γ�B2A��B2A2���ֻ����B��D��G������������Ӧˮ��������֮�䶼�ܷ�Ӧ��D��F��Gԭ������������֮�͵���15��
A��B��D��E��F��G��ԭ������������������ֶ�����Ԫ�أ�A��B���γ�B2A��B2A2���ֻ����B��D��G������������Ӧˮ��������֮�䶼�ܷ�Ӧ��D��F��Gԭ������������֮�͵���15���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | V���ռ���Һ��/mL | V��HCl��/mL | |
| �ζ�ǰ���� | �ζ������ | ||
| 1 | 20.00 | 2.50 | 36.50 |
| 2 | 20.00 | 1.00 | 35.04 |
| 3 | 20.00 | 2.10 | 36.18 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
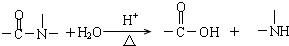 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������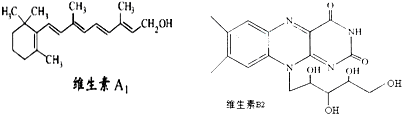
| A��1molά����A1�������ˮ�е�4molBr2�����ӳɷ�Ӧ |
| B��ά����A1�ķ���ʽΪC19H30O����һ��������ˮ�ĸ߷��� |
| C��ά����B2�����������³���ˮ���õ����л��������ڷ��������ö����� |
| D����-C4H9ȡ��ά����B2�����ϵ�һ��Hԭ�ӣ����ɵ�4��ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������£�PbSO4������pH=7��CH3COONH4��Һ��˵����CH3COO��2Pb��������� |
| B����Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�����ݲ�������Ksp��BaCO3����Ksp��BaSO4�� |
| C��DZͧ�ϵĺ˷�Ӧ��ʹ��Һ̬��-�ƺϽ������Ƚ��ʣ����Ͻ���n��Na����n��Al��������Ͷ�뵽������ˮ�пɵ���ɫ����Һ |
| D������֧ʢ��KI3��Һ���Թ��У��ֱ�μӵ�����Һ��AgNO3��Һ��ǰ����Һ�����������л�ɫ������˵��KI3��Һ�д���ƽ�⣺I3-�TI2+I- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A�����������У�����ʱ�õ���60% H2SO4���ܶ���1.5 g/cm3����������100 mL����H2SO4��Һ������Ҫ18.4 mol?L-1��Ũ����ԼΪ49.9mL |
| B������������H2O2��ֻ��Fe��OH��3�������֣�����Һ��c��Fe3+��=2.6��10-15mol?L-1������Һ��c��Cu2+����2.2��10-4mol?L-1 |
| C��������NH4HCO3�����ɵij�����Zn5��OH��6��CO3��2����÷�ӦΪ5ZnSO4+10NH4HCO3=Zn5��OH��6��CO3��2��+5��NH4��2SO4+8CO2��+2H2O |
| D�������ɵij�������̬��ΪZna��OH��b ��CO3��c�ģ�a��b��c���������������ּ�ʽ̼��п�Ļ�����ֱ������Zn5��OH��6��CO3��2 ��Zn3��OH��6CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com