
���۷��ɸĽ��л��߷��ӻ���������ʣ��߷��Ӿۺ���P�ĺϳ�·�����£�
��1��A�Ľṹ��ʽΪ________________
��2��C������Ϊ
��3��I��F���١��ۺϳɣ�F����ʹ��ˮ��ɫ.
a���ٵĻ�ѧ����ʽ��
b���ڵķ�Ӧ�Լ���
c���۵ķ�Ӧ������
��4������˵����ȷ����
a��C����ˮ����������
b��A��1,3-����ϩ��Ϊͬϵ��
c����I����Mʱ��1mol�������3molNaOH
d��N������˳���칹��
��5���߾���P����ˮ�ԣ�����E�γɵľۺ���__________���ǿ��������.
��6��E��N�������ʵ���֮��Ϊ1:1������������P��P�Ľṹ��ʽΪ
��7��E�ж���ͬ���칹�壬���������������칹���� �֣�������˳���칹����д�����е�һ��
a��������ֻ��һ�ֻ�״�ṹ
b��������������ȡ����
c��1mol���л�������ˮ��Ӧʱ������4molBr2
��1��CH2=CH2
��2���Ҷ���
(3)a��BrCH2CH=CHCH2Br+ 2NaOH HOCH2CH=CHCH2OH+ 2NaBr
HOCH2CH=CHCH2OH+ 2NaBr
b���ӳɷ�Ӧ
c��������Ӧ
��4��a��c
��5��ǿ
��6�� 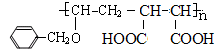
(7 ) 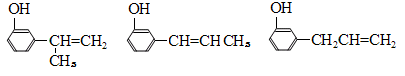
�������������������������֪A�����ӳɡ�ˮ�⡢��ˮ���ѣ���֪����������ˮ��ȥ����E������A��B��C��D��E�ֱ�ΪCH2=CH2��CH2BrCH2Br��CH2OHCH2OH�� 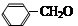 ��
��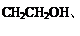
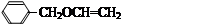
������������������̣�1,3-����ϩ����1,4-�ӳɣ�ˮ�⡢�ӳ�HCl������̼̼˫��������������֪����������ȥ�Ȳ���õ�M������F��G��H��M��N�ֱ�ΪBrCH2CH=CHCH2Br��HOCH2CH=CHCH2OH��HOCH2CH2CHClCH2OH��NaOOCCH=CHCOONa��HOOCCH=CHCOOH��(3)F ����̼̼˫�����ٵĻ�ѧ����ʽ��BrCH2CH=CHCH2Br+ 2NaOH  HOCH2CH=CHCH2OH+ 2NaBr����4�� a��CΪ�Ҷ�������ˮ���ܣ���ȷ��b��AΪ��ϩ�ǵ�ϩ����1,3-����ϩ�Ƕ�ϩ������������Ŀ��ͬ������ͬϵ����� c.I����2���Ȼ���1����ԭ��ˮ������HCl����1molI�������3molNaOH����ȷ��d.N̼̼˫�����˵�̼ԭ���������ֲ�ͬ��ԭ�ӣ��ʴ���˳���칹�壬����5��P�к�����ˮ���Ȼ���������ˮ����ǿ������ˮ����E�γɵľۺ����6��N��F�ۺϵõ�
HOCH2CH=CHCH2OH+ 2NaBr����4�� a��CΪ�Ҷ�������ˮ���ܣ���ȷ��b��AΪ��ϩ�ǵ�ϩ����1,3-����ϩ�Ƕ�ϩ������������Ŀ��ͬ������ͬϵ����� c.I����2���Ȼ���1����ԭ��ˮ������HCl����1molI�������3molNaOH����ȷ��d.N̼̼˫�����˵�̼ԭ���������ֲ�ͬ��ԭ�ӣ��ʴ���˳���칹�壬����5��P�к�����ˮ���Ȼ���������ˮ����ǿ������ˮ����E�γɵľۺ����6��N��F�ۺϵõ�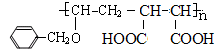 ��
��
��7�����ǵ���ͬ���칹���о߱����������������к��з��ǻ�����Ӧ�����ڶ�λ����λ�ñ���ȡ������Ӧ��1��̼̼˫������������3�֣�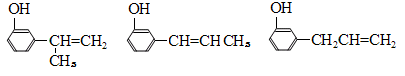
���㣺�л��ϳ��⣬�����л���֮����ܱ䣬�漰�����ŵ����ʡ���Ӧ���͡�����ʽ��д��ͬ���칹����жϵ��й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������Է�����AΪԭ���Ʊ�������(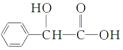 )������ͼ����ش��������⣺
)������ͼ����ش��������⣺
(1)A�Ľṹ��ʽΪ________��D�й����ŵ�������________��
(2)C������̼ԭ��________(��ܡ����ܡ�)���棬����B�Ľṹ��ʽ���������֣��ֱ�Ϊ________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
E��F��________________________________________________________��
G��H��_______________________________________________________��
(4)����ת�������ڼӳɷ�Ӧ����________(�����)��
(5)���ʵ��֤������B������Ԫ��________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���f�������һ��������ʹ��ҩ����Ĺ�ҵ�ϳ�·�����£�
��ش��������⣺
��1��A���ڱ�¶�ڿ����л���ʣ���ԭ���� ��
��2��A��B�ķ�Ӧͨ���ڵ���ʱ���С��¶�����ʱ��������ȡ������������ࡣ���ж�����ȡ�����У���������ɵ��� ��������ĸ��
a�� b��
b�� c��
c�� d��
d��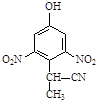
��3����E������ͬ���칹���У���������̼ԭ�ӵķ����� ��������ĸ��
a��
b��
c��
d��
��4��F�Ľṹ��ʽ ��
��5��D��ͬ���칹��H��һ�֦�-�����ᣬH�ɱ�����KMnO4��Һ�����ɶԱ������ᣬ��H�Ľṹ��ʽ�� ���߾���L��Hͨ���ļ����Ӷ��ɣ�L�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I��ç��������ЧӦ��H5N1�������в�����ҩ���ơ�����Ҫ�ɷ֡�
��1��ç�����пɷ����ӳɷ�Ӧ�Ĺ�����Ϊ______ (�����ƣ���
��2�������ʵ�����ç���ᡢ�ס��ҷֱ���NaOH��Һ��ȫ��Ӧ������NaOH�����ʵ���֮��Ϊ______��
��3��д����������������ç�����ͬ���칹��Ľṹ��ʽ____________��
��i����״�ṹ���������⣬����������
��ii������NaOH��Һ��Ӧ��
II��PETΪ�ۺ������������Ȫˮ����ƿ��PET������������ͼ��
��֪��A��B��C��D��E���DZ��Ķ�λ��Ԫȡ���AΪ����E���ڱ������ụΪͬ���칹�塣
��4��B��C�ķ�Ӧ����Ϊ______��
��5���ܼ���C��D���Լ���______��
��6��E���Ҷ�����Ӧ����PET�Ļ�ѧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ú�ġ���������ʹú��������Դ����Ч;��֮һ������Ҫ��ӦΪ��C+H2O CO��+H2�������ᱽ����(F)���������Ϻ�ҩ�����Ҫԭ�ϡ���ͼ����úΪԭ�Ϻϳɼ��ᱽ������·��ͼ�����ַ�Ӧ����������������ȥ������D�ķ���ʽΪC9H10O�����ܷ���������Ӧ��
CO��+H2�������ᱽ����(F)���������Ϻ�ҩ�����Ҫԭ�ϡ���ͼ����úΪԭ�Ϻϳɼ��ᱽ������·��ͼ�����ַ�Ӧ����������������ȥ������D�ķ���ʽΪC9H10O�����ܷ���������Ӧ��
��������ת����ϵ�ش��������⣺
��1��д��A��D�Ľṹ��ʽ��A��________________________ D��__________________________
��2��D��E�ķ�Ӧ����Ϊ_____________��B�еĹ�������_____________��
��3��дɽ����B�еĹ����ŵ��Լ������ֵ�����
�Լ�_____________������_______________________________________��
��4��д��C��E��Ӧ����F�Ļ�ѧ����ʽ_______________________________________��
��5�����ϳ�����( CO��H2)�����ϳ��л����ԭ���⣬����������;����_____________������������
��6��F�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ��
���������࣬���ܷ���������Ӧ�� �ڱ����ϵ�һ��ȡ����ֻ�����ֽṹ��
�۷��ӽṹ��ֻ����������______________________________��______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������E��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ������ͨ����ͼ��ʾ��·�ߺϳɣ�
��1��A�к��еĹ���������Ϊ ��Cת��ΪD�ķ�Ӧ������ ��
��2��1mol E������ mol H2��Ӧ��д��D������NaOH��Һ��ȫ��Ӧ�Ļ�ѧ����ʽ�� ��
��3��д��ͬʱ��������������B��һ��ͬ���칹��Ľṹ��ʽ ��
A���ܹ�����������Ӧ��Ҳ�ܷ���ˮ�ⷴӦ
B��������ֻ�����ֲ�ͬ��ѧ������ԭ��
C������FeCl3��Һ������ɫ��Ӧ
��4������D�����NaOH��Һ���ȳ�ַ�Ӧ�������ɡ����գ���������в����Ĺ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������F(ƥ����͡)���ڸߵ��̴�Ѫ֢�����ƣ���ϳ�·�����£� ��1��������D�й����ŵ�����Ϊ �� ��������
��1��������D�й����ŵ�����Ϊ �� ��������
��2��A��B�ķ�Ӧ������
��3��д��ͬʱ��������������A��һ��ͬ���칹��Ľṹ��ʽ��
I�������к���������������.��������3�ֲ�ͬ��ѧ�������⣻��.����һO��Oһ��
��4��ʵ��D��E��ת���У�������X�ķ���ʽΪC19H15NFBr��д����ṹ��ʽ�� ��
��5����֪��������E��CF3COO H����������ת��Ϊ ����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� ��
����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� ��
��6�������ϳ�·���У�����۵IJ����D����� ���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����
���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д���� Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�CH3CH2OH
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�CH3CH2OH CH2��CH2
CH2��CH2 CH3CH3
CH3CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������M��һ���������ಡҩ����м��壬��AΪԭ�ϵĹ�ҵ�ϳ�·������ͼ��ʾ��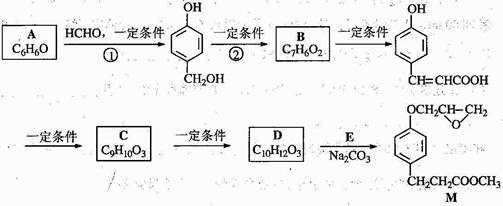
��֪��RONa+ R��X��ROR��+ NaX
�����������������գ�
��1��д����Ӧ���͡���Ӧ�� ��Ӧ��
��2��д���ṹ��ʽ��A C
��3��д��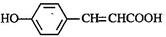 ����λ�칹���������ˮ�����㶹�صĽṹ��ʽ ��
����λ�칹���������ˮ�����㶹�صĽṹ��ʽ ��
��4����C����D����һ����Ӧ���� ����Ӧ������ ��
��5��д����D����M�Ļ�ѧ��Ӧ����ʽ ��
��6��AҲ���Ʊ�������(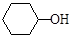 )��ԭ�ϣ�д������A����ȫת��Ϊ�������ķ�����
)��ԭ�ϣ�д������A����ȫת��Ϊ�������ķ�����
_______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�ҹ�������Ա����Ȼ����ֲ��������һ�־�������ɱ�������ס��ⶾ���õĻ�����H��H�ĺϳ�·�����£�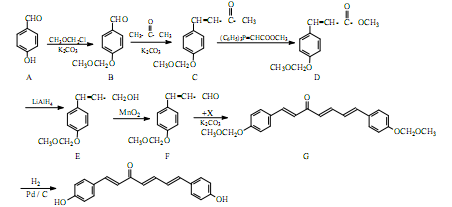
��1��A��B�ķ�Ӧ������ ��
��2��������B�еĺ���������Ϊ �� ������������ƣ���
��3��д��ͬʱ��������������B��һ��ͬ���칹��Ľṹ��ʽ ��
I��������������ȡ������II����������6�ֲ�ͬ��ѧ�������⣻III��������FeCl3��Һ������ɫ��Ӧ�����ܷ���������Ӧ��ˮ�����֮һҲ�ܷ���������Ӧ��
��4��ʵ��F��G��ת���У�����Ļ�����X��C12H14O3���Ľṹ��ʽΪ ��
��5��������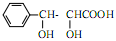 �Ǻϳ���ɼ���Ĺؼ��������д����
�Ǻϳ���ɼ���Ĺؼ��������д����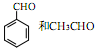 Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�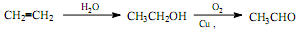
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com