
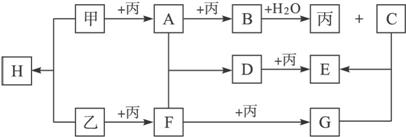
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗ±ū.___________£¬H. ___________”£
£Ø2£©Š“³öĻĀĮŠ±ä»ÆµÄĄė×Ó·½³ĢŹ½
B+H2O![]() ±ū+C£ŗ__________________________________________________”£
±ū+C£ŗ__________________________________________________”£
£Ø3£©ŅŅ+±ū![]() FŹĒ¹¤ŅµÉĻµĆµ½FµÄ·“Ó¦Ö®Ņ»£¬ĒėŠ“³ö¹¤ŅµÉĻµĆµ½FµÄĮķŅ»»Æѧ·“Ó¦·½³ĢŹ½£ŗ______
FŹĒ¹¤ŅµÉĻµĆµ½FµÄ·“Ó¦Ö®Ņ»£¬ĒėŠ“³ö¹¤ŅµÉĻµĆµ½FµÄĮķŅ»»Æѧ·“Ó¦·½³ĢŹ½£ŗ______
_____________________________________________________________________ӣ
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| Ź±¼ä£Øs£© | 0 | 1 | 2 | 3 | 4 | 5 |
| n£ØF£©/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ąż
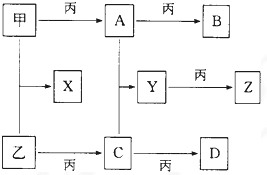
ŅŃÖŖ¼×”¢ŅŅ”¢±ūĪŖ³£¼ūµ„ÖŹ£¬A”¢B”¢C”¢D”¢E”¢F”¢G”¢X¾łĪŖ³£¼ūµÄ»ÆŗĻĪļ£»BŗĶXµÄĦ¶ūÖŹĮæĻąĶ¬£¬EµÄĻą¶Ō·Ö×ÓÖŹĮæ±ČDµÄĻą¶Ō·Ö×ÓÖŹĮæ“ó16£¬ŌŚŅ»¶ØĢõ¼žĻĀ£¬ø÷ĪļÖŹĻą»„×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ŗ

(1)Š“³öXµÄµē×ÓŹ½ŗĶGµÄ»ÆѧŹ½£ŗX__________£¬G__________£»
(2)Š“³öÓŠ¹Ų±ä»ÆµÄ»Æѧ·½³ĢŹ½£ŗ
B£«H2O£ŗ______________________________£»
D£«±ū£ŗ______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2009-2010Ń§ÄźÕż¶Ø֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ£ØĄķæĘ£© ĢāŠĶ£ŗŃ”ŌńĢā
£Ø6·Ö£©ŅŃÖŖ¼×”¢ŅŅ”¢±ūĪŖ³£¼ūµ„ÖŹ£¬A”¢B”¢C”¢D”¢E”¢F”¢G”¢X¾łĪŖ³£¼ūµÄ»ÆŗĻĪļ£»BŗĶXµÄĦ¶ūÖŹĮæĻąĶ¬£¬EµÄĻą¶Ō·Ö×ÓÖŹĮæ±ČDµÄĻą¶Ō·Ö×ÓÖŹĮæ“ó16£¬ŌŚŅ»¶ØĢõ¼žĻĀ£¬ø÷ĪļÖŹĻą»„×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ŗ

¢Å Š“³öXµÄµē×ÓŹ½ŗĶGµÄ»ÆѧŹ½£ŗX__________£¬G__________£»
¢Ę Š“³öÓŠ¹Ų±ä»ÆµÄ»Æѧ·½³ĢŹ½£ŗ
B£«H2O£ŗ______________________________£»
D£«±ū£ŗ______________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com