
��12�֣��ɼ������ӻ�������ɵĻ����������������е������֣�K����Cl����NH4+��Mg2����Ba2����CO32-��SO42-�����û��������ˮ��ó�����Һ����ȡ����100 mL����Һ�ֱ��������ʵ�飺
ʵ����� | ʵ������ | ʵ���� |
1 | ��AgNO3��Һ | �а�ɫ�������� |
2 | ������NaOH��Һ������ | �ռ�������1.12 L(������ɱ�״���µ����) |
3 | ������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.27 g���ڶ��γ�������Ϊ2.33 g�� |
�Իش��������⣺
��1������ʵ��1��Cl���Ƿ���ڵ��ж���________(�һ�����ڡ���һ�������ڡ�����ȷ����)������ʵ��1��3�ж�ԭ�������һ�������ڵ�������________��
��2����ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
�����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
|
|
|
|
��3����ȷ��K���Ƿ���ڣ�________���жϵ�������______________________________
19.��12�֣���1������ȷ����1�֣� Ba2����Mg2����2�֣�
��2��
�����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
SO42-��1�֣� | 0.1��2�֣� |
CO32-��1�֣� | 0.2��2�֣� |
��3�����ڣ�1�֣���Һ�п϶����ڵ�������NH4+��CO32-��SO42-�������㣬NH4+�����ʵ���Ϊ0.05 mol��CO32-��SO42-�����ʵ����ֱ�Ϊ0.02 mol��0.01 mol�����ݵ���غ㣬��K��һ�����ڣ�2�֣�
��������
����������������⣬Ba2+��SO42-���ɷ������ӷ�Ӧ����BaSO4����������߲��ܹ��森Ba2+��CO32-�ɷ������ӷ�Ӧ����BaCO3�����������Ҳ���ܹ��棬��һ�ݼ���AgNO3��Һ�г������������ܷ���Cl-+Ag+�TAgCl����CO32-+2Ag+�TAg2CO3����SO42-+2Ag+�TAg2SO4�������Կ��ܺ���Cl-��CO32-��SO42-��
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.05mol����NaOH��Һ���Ȳ��������ֻ����NH4+����û�г�������˵��һ��������Mg2+���ʿ�ȷ��һ������NH4+��һ��������Mg2+�����ݷ�ӦNH4++OH-�TNH3��+H2O������NH3Ϊ0.05mol���ɵ�NH4+ҲΪ0.05mol�������ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӣ������������Ϊ2.33g�����ֳ�����������ΪBaCO3�����ֳ�������������ΪBaSO4��������ӦCO32-+Ba2+�TBaCO3����SO42-+Ba2+�TBaSO4������ΪBaCO3+2HCl�TBaCl2+CO2��+H2O��ʹBaCO3�ܽ⣮�����Һ��һ������CO32-��SO42-��һ��������Ba2+����������֪BaSO4Ϊ2.33g�����ʵ���Ϊ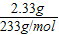 =0.01mol��BaCO3Ϊ6.27g-2.33g�T3.94g�����ʵ���Ϊ
=0.01mol��BaCO3Ϊ6.27g-2.33g�T3.94g�����ʵ���Ϊ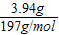 =0.02mol�������������ɵã���Һ��һ������CO32-��SO42-��NH4+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.02mol��0.01mol��0.04mol��һ��������Mg2+��Ba2+��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.05 mol��������Һ�е���غ㣬��֪K+һ�����ڣ�K+���ʵ�����0.01 mol����K+���ʵ�����0.01 molʱ����Һ�л����뺬��Cl-����K+���ʵ���=0.01 molʱ����Һ�в�����Cl-��
=0.02mol�������������ɵã���Һ��һ������CO32-��SO42-��NH4+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.02mol��0.01mol��0.04mol��һ��������Mg2+��Ba2+��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.05 mol��������Һ�е���غ㣬��֪K+һ�����ڣ�K+���ʵ�����0.01 mol����K+���ʵ�����0.01 molʱ����Һ�л����뺬��Cl-����K+���ʵ���=0.01 molʱ����Һ�в�����Cl-��
��1��ʵ��1����AgNO3��Һ�г������������ܷ���Cl-+Ag+�TAgCl����CO32-+2Ag+�TAg2CO3����SO42-+2Ag+�TAg2SO4�������Կ��ܺ���Cl-��CO32-��SO42-��ʵ��1����ȷ������Cl-����������������֪Cl-���ܺ��У�
������������֪����Һ��һ��������Mg2+��Ba2+����Ϊ������ȷ����Mg2+��Ba2+��
��2����������������֪����Һ��һ�����ڵ�������ΪCO32-��SO42-��
SO42-���ʵ���Ϊ0.01mol��SO42-���ʵ���Ũ��=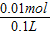 =0.1mol/L��
=0.1mol/L��
CO32-���ʵ���Ϊ0.02mol��CO32-���ʵ���Ũ��Ϊ 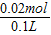 =0.2mol/L����Ϊ��
=0.2mol/L������
�����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
SO42-��1�֣� | 0.1��2�֣� |
CO32-��1�֣� | 0.2��2�֣� |
��3����Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.02mol��0.01mol��0.04mol��CO32-��SO42-��������ɷֱ�Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.05 mol��������Һ�е���غ㣬��֪K+һ�����ڣ���Ϊ�����ڣ���Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.02mol��0.01mol��0.04mol��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.05 mol��������Һ�е���غ㣬��֪K+һ�����ڡ�
���㣺�������ӵ��ж�����顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ӱ�ʡ��ɽ�и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��15%��NaOH��Һ������100 gˮ��õ�125 ml��������Ϊ30%��NaOH��Һ�����ʱ��Һ�����ʵ���Ũ��Ϊ( )
A��6 mol��L-1 B��6.25 mol��L-1 C��6.75 mol��L-1 D��7 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ӱ�ʡ�����и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪a��N2 ���� b�����ӣ����ӵ���������ֵΪ��
A��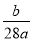 B��
B��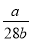 C��
C��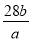 D��
D��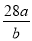
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ�����и�һ��ѧ�ڵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
ij��Һ�к��нϴ�����Cl-��CO32-��OH- 3�������ӣ����ֻȡһ�θ���Һ�ܹ��ֱ��������������μ������������ʵ�����˳����ȷ���ǣ� ��
�ٵμӹ���Mg(NO3)2��Һ �ڹ��� �۵μ�AgNO3��Һ �ܵμӹ���Ba(NO3)2��Һ
A���٢ڢܢڢ� B���ܢڢ٢ڢ� C���٢ڢۢڢ� D���ܢڢۢڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ�����и�һ��ѧ�ڵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
����˵����ȷ���� ( )
A����������ָ��������в�����Ԫ�ص���,��HCl��CH4��
B�������ܽ��ԵIJ�ͬ,�ɽ����Ϊ�����Լ�Ϳ����Լ�
C�����ᷴӦ�����κ�ˮ������һ���Ǽ���������
D�����е����������ﶼ������ˮ���ϵõ���Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ�������һ��ѧ�����л�ѧ�������Ծ��������棩 ���ͣ�ѡ����
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ��ݴ�����˵����ȷ����
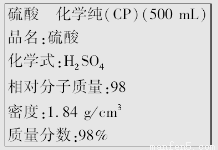
A������������ʵ���Ũ��Ϊ9.2 mol/L
B��1 mol Zn�������ĸ����ᷴӦ����2 g����
C������200 mL 4.6 mol/L��ϡ������ȡ������50 mL
D����������ˮ���������������Һ�����ʵ���Ũ�ȴ���9.2 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ�������һ��ѧ�����л�ѧ�������Ծ��������棩 ���ͣ�ѡ����
���������ܵ��������ڵ���ʵ���
A��ʯī B��NaCl��Һ C���Ȼ��ƹ��� D�����ڵ�NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ�������и߶���ѧ�����л�ѧ�������Ծ��������棩 ���ͣ�ѡ����
��4 mol A�����2 mol B������2 L�������л�ϲ���һ�������·������·�Ӧ��
2A������+B������ 2C������������2 s���룩����C��Ũ��Ϊ0.6 mol��L��1���������м���˵����
2C������������2 s���룩����C��Ũ��Ϊ0.6 mol��L��1���������м���˵����
��������A��ʾ�ķ�Ӧƽ������Ϊ0.3 mol��L��1��s��1
��������B��ʾ�ķ�Ӧ��ƽ������Ϊ0.6 mol��L��1��s��1
��2sʱ����A��ת����Ϊ70%
��2sʱ����B��Ũ��Ϊ0.7 mol��L��1
������ȷ����
A.�٢� B.�٢� C.�ڢ� D.�ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��C mol/L��AlCl3��ҺV1 mL��ˮϡ����V2mL��ϡ�ͺ���Һ��Cl-���ʵ���Ũ��Ϊ ( )
A��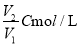 B��
B��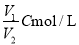 C��
C��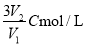 D��
D��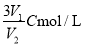
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com