

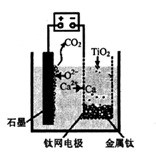
 2Ca+O2”ü£¬2Ca+ TiO2="Ti" +2CaO£¬æÉÖŖCaOµÄ×ÜĮæ²»»į±ä(3·Ö) £ØŗĻĄķ“š°øøų·Ö£©
2Ca+O2”ü£¬2Ca+ TiO2="Ti" +2CaO£¬æÉÖŖCaOµÄ×ÜĮæ²»»į±ä(3·Ö) £ØŗĻĄķ“š°øøų·Ö£©

 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
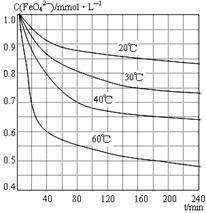
| A£®ÕōĮó·ØŹĒŗ£Ė®µ»ÆµÄ·½·ØÖ®Ņ» |
| B£®µĖ®µÄĆܶȊ”ÓŚŗ£Ė®µÄĆÜ¶Č |
| C£®ČŚ»ÆµÄŃ©Ė®ÖŠæóĪļÖŹŗ¬Įæ±ČÉī¾®Ė®ÖŠµÄÉŁ |
| D£®0 ”ęŅŌÉĻ£¬ĪĀ¶ČŌ½øߣ¬Ė®µÄĆܶČŌ½Š” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

 2I£+S4O62££©
2I£+S4O62££©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ź³ŃĪ”¢µĖ® | B£®ÄĘ”¢Ć¾ | C£®ÉÕ¼ī”¢ĀČĘų | D£®ä唢µā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
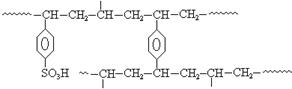
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
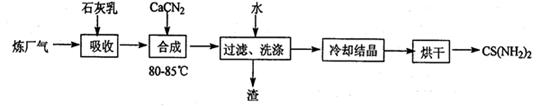
 2H2(g)+S2(g),ĘäĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK=”£
2H2(g)+S2(g),ĘäĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK=”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ė®ĆŗĘų | B£®Ņ»Ńõ»ÆĢ¼ | C£®ĢģČ»Ęų | D£®ĒāĘų |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com