
| ±ąŗÅ | ŠŌÖŹ | »Æѧ·½³ĢŹ½ |
| 1 | »¹ŌŠŌ | H2SO3+Br2+2H2OØTH2SO3+2HBr |
| 2 | ĖįŠŌ | H2SO3+2NaOH=Na2SO3+2H2O |
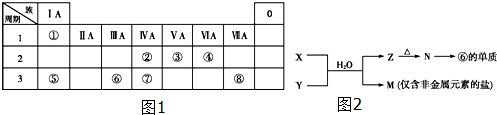
·ÖĪö ÓÉŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆæÉÖŖ£ŗ¢ŁĪŖHŌŖĖŲ”¢¢ŚĪŖCŌŖĖŲ”¢¢ŪĪŖNŌŖĖŲ”¢¢ÜĪŖOŌŖĖŲ”¢¢ŻĪŖNaŌŖĖŲ”¢¢ŽĪŖAlŌŖĖŲ”¢¢ßĪŖSiŌŖĖŲ”¢¢ąĪŖSŌŖĖŲ£¬
£Ø1£©ÓÉO”¢Na”¢ClŠĪ³ÉµÄ¼Čŗ¬Ąė×Ó¼üÓÖŗ¬¹²¼Ū¼ü»ÆŗĻĪļĪŖNaClOµČ£»
£Ø2£©ŗ¬ŌŖĖŲ¢ąµÄµ„ÖŹĪŖĀČĘų£¬¾ßÓŠĒæŃõ»ÆŠŌ£¬æÉŅŌĻø¾śĻū¶¾£¬¹ŹŃ”ĻīÖŠ¾ßÓŠĒæŃõ»ÆŠŌµÄĪļÖŹæÉŅŌĢę“śĀČĘų£»
£Ø3£©WÓė¢ÜŹĒĻąĮŚµÄĶ¬Ö÷×åŌŖĖŲ£¬ÓÉĶ¼æÉÖŖ£¬¢ÜŹĒŃõŌŖĖŲ£¬¹ŹWĪŖĮņŌŖĖŲ£¬H2SO3¾ßÓŠĖįŠŌ”¢»¹ŌŠŌ”¢Ńõ»ÆŠŌ£¬Óėäå·“Ó¦Éś³ÉĮņĖįÓėHBr±ķŹ¾»¹ŌŠŌ£¬ÓėĒāŃõ»ÆÄĘ·“Ó¦±ķĻÖĖįŠŌ£»
£Ø4£©MŹĒ½öŗ¬·Ē½šŹōµÄŃĪ£¬ĖłŅŌŅ»¶ØŹĒļ§ŃĪ£¬¢ŽĪŖAlŌŖĖŲ£¬ĖłŅŌĶʶĻNŹĒAl2O3£¬ZĪŖAl£ØOH£©3£¬ČōMŗ¬ÓŠClŌŖĖŲ£¬ŌņXĪŖAlCl3£¬YĪŖNH3£¬MĪŖNH4Cl£®
½ā“š ½ā£ŗÓÉŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆæÉÖŖ£ŗ¢ŁĪŖHŌŖĖŲ”¢¢ŚĪŖCŌŖĖŲ”¢¢ŪĪŖNŌŖĖŲ”¢¢ÜĪŖOŌŖĖŲ”¢¢ŻĪŖNaŌŖĖŲ”¢¢ŽĪŖAlŌŖĖŲ”¢¢ßĪŖSiŌŖĖŲ”¢¢ąĪŖSŌŖĖŲ£®
£Ø1£©ÓÉO”¢Na”¢ClŠĪ³ÉµÄ¼Čŗ¬Ąė×Ó¼üÓÖŗ¬¹²¼Ū¼ü»ÆŗĻĪļĪŖNaClOµČ£¬¹Ź“š°øĪŖ£ŗNaClO£»
£Ø2£©ŗ¬ŌŖĖŲ¢ąµÄµ„ÖŹĪŖĀČĘų£¬¾ßÓŠĒæŃõ»ÆŠŌ£¬æÉŅŌĻø¾śĻū¶¾£¬¹ŹŃ”ĻīÖŠ¾ßÓŠĒæŃõ»ÆŠŌµÄĪļÖŹæÉŅŌĢę“śĀČĘų£¬Ń”ĻīÖŠClO2”¢K2FeO4¾ßÓŠĒæŃõ»ÆŠŌ£¬
¹č“š°øĪŖ£ŗAC£»
£Ø3£©WÓė¢ÜŹĒĻąĮŚµÄĶ¬Ö÷×åŌŖĖŲ£¬ŌņWĪŖSŌŖĖŲ£¬H2SO3µÄ¾ßÓŠŃõ»ÆŠŌ”¢»¹ŌŠŌ”¢ĖįŠŌ”¢²»ĪČ¶ØŠŌµČ£¬æÉŅŌ±»ĒæŃõ»Æ¼ĮŃõ»Æ£¬ČēH2SO3+Br2+2H2O=H2SO3+2HBr£¬ÓėNaOH·¢ÉśÖŠŗĶ·“Ó¦H2SO3+2NaOH=Na2SO3+2H2O£¬
¹Ź“š°øĪŖ£ŗ
| 1 | »¹ŌŠŌ | H2SO3+Br2+2H2OØTH2SO3+2HBr |
| 2 | ĖįŠŌ | H2SO3+2NaOH=Na2SO3+2H2O |
µćĘĄ ±¾Ģāæ¼²éŌŖĖŲÖÜĘŚ±ķÓėĪŽ»śĪļµÄĶʶĻ£¬Éę¼°ŌŖĖŲ»ÆŗĻĪļŠŌÖŹ”¢ŹµŃé·½°øÉč¼Ę”¢Ąė×ÓÅØ¶Č“óŠ”±Č½ĻµČ£¬×¢Ņā¶Ō»ł“”ÖŖŹ¶µÄ»żĄŪÕĘĪÕ£¬ÄѶČÖŠµČ£®


 Š”ѧ½Ģ²ÄČ«²āĻµĮŠ“š°ø
Š”ѧ½Ģ²ÄČ«²āĻµĮŠ“š°ø Š”ѧŹżŃ§æŚĖćĢāæØĶŃæŚ¶ų³öĻµĮŠ“š°ø
Š”ѧŹżŃ§æŚĖćĢāæØĶŃæŚ¶ų³öĻµĮŠ“š°ø ÓÅŠćÉśÓ¦ÓĆĢāæØæŚĖćĢģĢģĮ·ĻµĮŠ“š°ø
ÓÅŠćÉśÓ¦ÓĆĢāæØæŚĖćĢģĢģĮ·ĻµĮŠ“š°ø Õć½Ö®ŠĒæĪŹ±ÓÅ»Æ×÷ŅµĻµĮŠ“š°ø
Õć½Ö®ŠĒæĪŹ±ÓÅ»Æ×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ńõ»ÆĀĮµÄČŪµćŗÜøߣ¬ĖłŅŌ²»æÉŅŌÓĆĄ“Ņ±Į¶ĀĮ | |
| B£® | ĒāŃõ»ÆĀĮŹĒŅ»ÖÖ½ŗד³Įµķ£¬ÓŠ½Ļ“ó±ķĆ껿£¬ÓŠĪüø½ŠŌ£¬æÉÓĆ×÷¾»Ė®¼Į | |
| C£® | ŹµŃéŹŅæÉŅŌÓĆĒāŃõ»ÆÄĘÓėĀČ»ÆĀĮĄ“ÖʱøĒāŃõ»ÆĀĮ | |
| D£® | ĒāŃõ»ÆĀĮ¼ČæÉÓėĒæĖį·“Ó¦ÓÖæÉÓėĒæ¼ī·“Ó¦£¬ŹĒĮ½ŠŌĒāŃõ»ÆĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±łÓėĖ®¹²“ęĪļŹōÓŚ»ģŗĻĪļ | B£® | ±łµÄĆܶȱČĖ®“ó | ||
| C£® | ±łÓė¶žŃõ»Æ¹čµÄ¾§ĢåĄąŠĶĻąĖĘ | D£® | Ēā¼üŌŚ±ł¾§Ģå½į¹¹ÖŠĘš¹Ų¼ü×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com