
���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣50 mL 0.50 mol/L������50 mL 0.55 mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
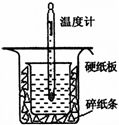
��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ�� ������֮�⣬װ���е�һ�����Դ����� ��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ�� ________ (� ƫ��ƫС������Ӱ�족)��
������к͵ζ�����ѧ��ѧ����ʵ�顣ijѧУ��ѧ����С���ñ�Һ����ζ�δ֪Ũ�ȵ�����������Һ���Իش��������⡣
��1���ζ������У��۾�Ӧע�� ��
��2��������̨�ϵ�һ�Ű�ֽ����Ŀ���� ��
��2��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ����� | ����NaOH��Һ�����/mL | 0.1000 mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ϱ�������ʽ��������NaOH��Һ�����ʵ���Ũ��
��
��4������ʵ������Եζ��������ʲôӰ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)?
a.�۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ���� ��
b.������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00 mL����Һ����ζ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ������ʮһ���и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��9�֣�
���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣50 mL 0.50 mol/L������50 mL 0.55 mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺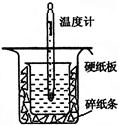
��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ�� ������֮�⣬װ���е�һ�����Դ����� ��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ�� ________ (� ƫ��ƫС������Ӱ�족)��
������к͵ζ�����ѧ��ѧ����ʵ�顣ijѧУ��ѧ����С���ñ�Һ����ζ�δ֪Ũ�ȵ�����������Һ���Իش��������⡣
��1���ζ������У��۾�Ӧע�� ��
��2��������̨�ϵ�һ�Ű�ֽ����Ŀ���� ��
��2��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ����� | ����NaOH��Һ�����/mL | 0.1000 mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013�꺣�������λ���ѧ�߶��ϸ��н�ѧ�����������ѧ�Ծ����������� ���ͣ������
��10�֣�ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β����������0.5mol�� L-1���ᡢ0.55mol�� L-1NaOH��Һ��
��ȱ�ٵ�ʵ�鲣����Ʒ�� �� ��
��2��ʵ�����ܷ��û���ͭ˿��������滷�β����������
����ܡ�������ԭ���� ��
��3�����Ǽ�¼��ʵ���������£�
| ʵ �� �� Ʒ | �� Һ �� �� | �к��� ��H | |||
| t1 | t2 | ||||
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� | |
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�켪��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣�
���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣50 mL 0.50 mol/L������50 mL 0.55 mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺

��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ�� ������֮�⣬װ���е�һ�����Դ����� ��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ�� ________ (� ƫ��ƫС������Ӱ�족)��
������к͵ζ�����ѧ��ѧ����ʵ�顣ijѧУ��ѧ����С���ñ�Һ����ζ�δ֪Ũ�ȵ�����������Һ���Իش��������⡣
��1���ζ������У��۾�Ӧע�� ��
��2��������̨�ϵ�һ�Ű�ֽ����Ŀ���� ��
��2��ijѧ������3��ʵ��ֱ��¼�й��������±���
|
����� |
����NaOH��Һ�����/mL |
0.1000 mol/L��������/mL |
||
|
�ζ�ǰ�̶� |
�ζ���̶� |
��Һ���/mL |
||
|
��һ�� |
25.00 |
0.00 |
26.11 |
26.11 |
|
�ڶ��� |
25.00 |
1.56 |
30.30 |
28.74 |
|
������ |
25.00 |
0.22 |
26.31 |
26.09 |
�����ϱ�������ʽ�������NaOH��Һ�����ʵ���Ũ��
��
��4������ʵ������Եζ��������ʲôӰ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)?
a.�۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ���� ��
b.������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00 mL����Һ����ζ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com