

 Ba+CO2������Ԫ�ػ��ϼ�δ�����仯�����ڷֽⷴӦ����Ӧ��Ϊ2BaO+O2
Ba+CO2������Ԫ�ػ��ϼ�δ�����仯�����ڷֽⷴӦ����Ӧ��Ϊ2BaO+O2 2BaO2����Ԫ�ػ��ϼ۷����仯������������ԭ��Ӧ����Ӧ��BaO2+H2SO4=BaSO4��+H2O2����Ԫ�ػ��ϼ�δ�����仯�����ڸ��ֽⷴӦ������7����Ӧ�Т٢ڢ�����������ԭ��Ӧ���ʴ�Ϊ��3��
2BaO2����Ԫ�ػ��ϼ۷����仯������������ԭ��Ӧ����Ӧ��BaO2+H2SO4=BaSO4��+H2O2����Ԫ�ػ��ϼ�δ�����仯�����ڸ��ֽⷴӦ������7����Ӧ�Т٢ڢ�����������ԭ��Ӧ���ʴ�Ϊ��3��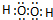 ��
��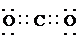 ��
�� ��
��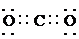 ��
��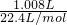 =0.045mol��������Һ������������ʵ���Ϊ0.05mol-0.045mol=0.005mol����Һ������������ʵ���Ũ��Ϊ
=0.045mol��������Һ������������ʵ���Ϊ0.05mol-0.045mol=0.005mol����Һ������������ʵ���Ũ��Ϊ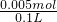 =0.05mol/L��
=0.05mol/L�� =0.2L=200mL���ʴ�Ϊ��200��
=0.2L=200mL���ʴ�Ϊ��200��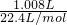 =0.045mol��������Һ������������ʵ���Ϊ0.05mol-0.045mol=0.005mol���ٸ���c=
=0.045mol��������Һ������������ʵ���Ϊ0.05mol-0.045mol=0.005mol���ٸ���c= ���������Ũ�ȣ�
���������Ũ�ȣ�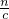 ���㣮
���㣮


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
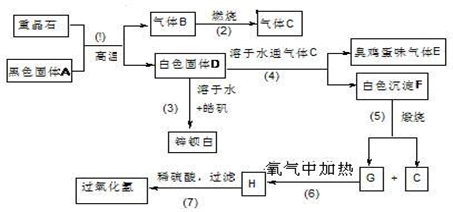
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4•7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ��ϰѧУ��������������������ۺϻ�ѧ���� ���ͣ������
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4?7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��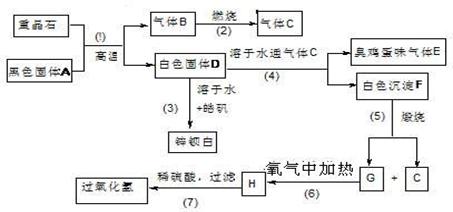
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4��Һ�У� �ų�H2S ����1008mL��������ɱ�״����
�ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ��������������������ۺϻ�ѧ���� ���ͣ������
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4•7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��
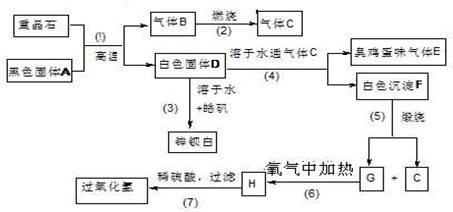
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4??7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��

��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com