
·ÖĪö £Ø1£©½į¹¹ĻąĖĘ£¬·Ö×Ó×é³ÉĻą²īnøöCH2Ō×ÓĶŵÄÓŠ»śĪļ»„ĪŖĶ¬ĻµĪļ£»
¾ßÓŠĻąĶ¬·Ö×ÓŹ½”¢²»Ķ¬½į¹¹µÄÓŠ»śĪļ»„ĪŖĶ¬·ÖŅģ¹¹Ģ壻
Ķ¬ÖÖŌŖĖŲµÄ²»Ķ¬µ„ÖŹ£¬»„ĪŖĶ¬ĖŲŅģŠĪĢ壻
¾ßÓŠĻąĶ¬ÖŹ×ÓŹż”¢²»Ķ¬ÖŠ×ÓŹżµÄŌ×ÓĪŖĶ¬Ī»ĖŲ£»
·Ö×ÓŹ½ĻąĶ¬”¢½į¹¹ĻąĶ¬µÄĪļÖŹĪŖĶ¬Ņ»ÖÖĪļÖŹ£»
£Ø2£©µ„Ļ©ĢžÓėHBr¼Ó³É·“Ó¦µÄ²śĪļÖ»ÓŠŅ»ÖÖ½į¹¹£¬ĖµĆ÷½į¹¹¶Ō³Ę£®
½ā“š ½ā£ŗ¢ŁO2””¢ŚČżĀČ¼×Ķé””¢ŪCH3CH2CH2OH””¢ÜO3””¢ŻCHCl3 ¢ŽCH3OCH2CH3””¢ß${\;}_{6}^{12}$C¢ąCH3CH£ØOH£©CH3””¢į${\;}_{6}^{13}$C¢āCH3OH
ŅŌÉĻ×éĪļÖŹÖŠ£¬¢ŪCH3CH2CH2OHŗĶ¢āCH3OH”¢¢ąCH3CH£ØOH£©CH3ŗĶ¢āCH3OHµÄ½į¹¹ĻąĖĘ£¬·Ö×Ó×é³ÉĻą²īnøöCH2Ō×ÓĶŵÄÓŠ»śĪļ£¬ĖüĆĒ»„ĪŖĶ¬ĻµĪļ£»
¢ŪCH3CH2CH2OH”¢¢ŽCH3OCH2CH3”¢¢ąCH3CH£ØOH£©CH3¾ßÓŠĻąĶ¬·Ö×ÓŹ½”¢²»Ķ¬½į¹¹µÄÓŠ»śĪļ£¬¶žÕß»„ĪŖĶ¬·ÖŅģ¹¹Ģ壻
¢ŁO2¢ÜO3ĪŖĶ¬ÖÖŌŖĖŲµÄ²»Ķ¬µ„ÖŹ£¬»„ĪŖĶ¬ĖŲŅģŠĪĢ壻
¢ß${\;}_{6}^{12}$C ŗĶ¢į${\;}_{6}^{13}$CĪŖ¾ßÓŠĻąĶ¬ÖŹ×ÓŹż”¢²»Ķ¬ÖŠ×ÓŹżµÄŌ×Ó£¬¶žÕßĪŖĶ¬Ī»ĖŲ£»
¢ŚČżĀČ¼×Ķé¢ŻCHCl3 ¶¼ŹĒČżĀČ¼×Ķé£¬ČżĀČ¼×Ķé²»“ęŌŚĶ¬·ÖŅģ¹¹Ģ壬ĖłŅŌ¶žÕßĪŖĶ¬ÖÖĪļÖŹ£¬
¹Ź“š°øĪŖ£ŗ¢Ū¢ā»ņ¢ą¢ā£»¢Ū¢Ž¢ą£»¢Ł¢Ü£»¢ß¢į£»¢Ś¢Ż£»
£Ø2£©Ģ¼Ō×ÓŹżŠ”ÓŚ»ņµČÓŚ6µÄµ„Ļ©Ģž£¬ÓėHBr¼Ó³É·“Ó¦µÄ²śĪļÖ»ÓŠŅ»ÖÖ½į¹¹£¬¼Ó³É²śĪļÖ»ÓŠŅ»ÖÖĖµĆ÷Ģ¼Ģ¼Ė«¼üŌŚÖŠ¼ä£¬·ūŗĻĢõ¼žµÄµ„Ļ©ĢžÓŠ£ŗCH2=CH2”¢CH3CH=CHCH3”¢CH3CH2CH=CHCH2CH3£¬ĖłµĆĶéĢžµÄŅ»Ā±“śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ2ÖÖ£¬ĖµĆ÷ÓŠ2ÖÖĒāŌ×Ó£¬øĆĻ©ĢžĪŖCH3CH=CHCH3£¬Ćū³ĘĪŖ2-¶”Ļ©£¬¹Ź“š°øĪŖ£ŗ3£»2-¶”Ļ©£»CH3CH=CHCH3£®
µćĘĄ ±¾Ģāæ¼²é½Ļ×ŪŗĻ£¬Éę¼°ÓŠ»śĪļµÄ½į¹¹ÓėŠŌÖŹ”¢Ļą¹ŲøÅÄīµÄ±ęĪöĻ©Ģž¼Ó³É·“Ó¦µÄ¹ęĀÉŅŌ¼°Ķ¬·ÖŅģ¹¹ĢåµÄŹéŠ“£¬²ąÖŲæ¼²éѧɜĖ¼æ¼ĪŹĢāµÄČ«ĆęŠŌ£¬°ŃĪÕÓŠ»śĪļÖŠ¹ŁÄÜĶÅÓėŠŌÖŹµÄ¹ŲĻµĪŖ½ā“šµÄ¹Ų¼ü£¬ĢāÄæ×ŪŗĻŠŌĒ棬µ«ÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCHO | B£® | CH3CHO | C£® | CH3CH2CHO | D£® | CH3CH£ØCH3£©CHO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | X”¢Y”¢Z”¢WµÄŌ×Ó°ė¾¶ŅĄ“Ī¼õŠ” | |
| B£® | WÓėXŠĪ³ÉµÄ»ÆŗĻĪļÖŠÖ»ŗ¬Ąė×Ó¼ü | |
| C£® | Wŗ¬ŃõĖįµÄĖįŠŌŅ»¶Ø±ČZµÄŗ¬ŃõĖįµÄĖįŠŌĒæ | |
| D£® | ČōWÓėYµÄŌ×ÓŠņŹżĻą²ī5£¬Ōņ¶žÕߊĪ³É»ÆŗĻĪļµÄ»ÆѧŹ½Ņ»¶ØĪŖY2W3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{c£Ø{H}^{+}£©}{c£ØO{H}^{-}£©}$=1012µÄČÜŅŗÖŠ£ŗMg2+”¢Al3+”¢NO3-”¢Cl- | |
| B£® | Ź¹pHŹŌÖ½ĻŌĄ¶É«µÄČÜŅŗÖŠ£ŗNH4+”¢NO3-”¢SO42-”¢Na+ | |
| C£® | ÓÉĖ®µēĄėµÄc£ØH+£©=1”Į10-14mol•L-1µÄČÜŅŗÖŠ£ŗMg2+”¢K+”¢Cl-”¢NO3- | |
| D£® | pH=0µÄČÜŅŗÖŠ£ŗK+”¢Fe3+”¢SO42-”¢SCN- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®Ę仳Ģ¬Ō×ÓÓŠ7ÖÖÄÜĮæ²»Ķ¬µÄµē×Ó£»
£®Ę仳Ģ¬Ō×ÓÓŠ7ÖÖÄÜĮæ²»Ķ¬µÄµē×Ó£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
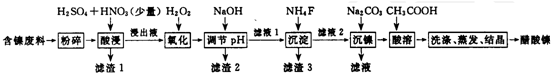
| ½šŹōĄė×Ó | æŖŹ¼³ĮµķµÄpH | ³ĮµķĶźČ«µÄpH | ĪļÖŹ | 20”ꏱČܽāŠŌ£ØH2O£© | |
| Fe3+ | 1.1 | 3.2 | CaS04 | Ī¢ČÜ | |
| Fe2+ | 5.8 | 8.8 | NiF | æÉČÜ | |
| Al3+ | 3.0 | 5.0 | CaF2 | ÄŃČÜ | |
| Ni2+ | 6.7 | 9.5 | NiCO3 | Ksp=9.6”Į10-4 |
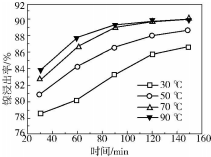
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | F2 | B£® | Cl- | C£® | NH3 | D£® | NH2- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com