
| Cu |
| �� |
| Cu |
| �� |
| m |
| M |
| m |
| M |
| 3.6g |
| 18g/mol |
| 6.6�� |
| 44g/mol |
| 1.6g |
| 16g/mol |
| Cu |
| �� |
| Cu |
| �� |


 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2SO4 |
| �� |
| H2SO4 |
| �� |
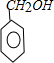
| ���� |
| �� |
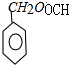 +H2O
+H2O
| ���� |
| �� |
 +H2O
+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 +HO-CH2CH2-OH
+HO-CH2CH2-OH| Ũ���� |
| �� |
 +2H2O
+2H2O +HO-CH2CH2-OH
+HO-CH2CH2-OH| Ũ���� |
| �� |
 +2H2O
+2H2O
| (�ӳɷ�Ӧ) |

| ||
| (ˮ�ⷴӦ) |

 +O2
+O2| Cu |
| �� |
 +2H2O��
+2H2O�� +O2
+O2| Cu |
| �� |
 +2H2O��
+2H2O��



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A������̼ԭ����������ԭ��������3�����䱽����ֻ��һ��ȡ��������ȡ����̼������֧����
��A����NaHCO3��Һ���ã�������ɫ���ݣ�
��A��һ�������¿������ᷢ��������Ӧ��
�����
(1)A�ķ���ʽ____________��A��һ�ֿ��ܵĽṹ��ʽ___________��A�к��������ŵ�����___________��
���л���A��������ת����ϵ(���¿�ͼ)����ݴ�ʱA�Ľṹ�ش�(2)��(3)��(4)�ʣ�

(2)��д����A��C��B��D�Ļ�ѧ��Ӧ����ʽ(ע����Ӧ����)����ע����Ӧ���ͣ�
A��C��____________________________________________����Ӧ���ͣ�___________��
B��D��____________________________________________����Ӧ���ͣ�___________��
(3)A��һ��ͬ���칹��F���䱽�������������ڵ�ȡ���������������Ȼ�����Һ������ɫ��Ӧ��F��ϡ���������ˮ������G��H������H�׳ơ�ľ�����������üپƹ���������ʧ��������������Ԫ�ס�
��д��F�Ľṹ��ʽ��___________________________________________________��
(4)��֪H��һ�������¿ɱ�������K��K��ˮ��Һ����������������д����
H��K�Ļ�ѧ����ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���½�������ģ���� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com