
��8�֣��״���CH3OH����һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϡ�
��֪��CH3OH(1) + O2(g)= CO(g) + 2H2O(g) ��H = ��443.64kJ��mol��1
2CO (g) + O2(g) = 2CO2(g) ��H =��566.0kJ��mol��1
��1����д��2molCH3OH(1)����������ȫȼ������CO2��H2O��g�����Ȼ�ѧ����ʽ��
��2��������Ա�½�������һ���ɼ״���������ǿ�����������Һ�������ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε磬�ݴ˻ش��������⣺
�״��� ����Ӧ��O2һ�������ĵ缫��Ӧ����ʽΪ
��3�����øõ����ij���������ͭ�������ƽ���������������6.4g�����������ļ״�������Ϊ g��
��1�� 2CH3OH(1) +3O2(g)= 2CO2(g) + 4H2O(g)����H =��1453.28kJ��mol��1
��2�� �� �����缫��Ӧ����ʽΪ O2+4e-+2H2O=4OH-
��3�� 1.07 g��
����:CH3OH(1) + O2(g) = CO(g) + 2H2O(g) ��H1= ��443.64 kJ��mol��1
2CO (g) + O2(g) = 2CO2(g) ��H 2=��566.0kJ��mol��1
2CH3OH(1) +3O2(g)= 2CO2(g) + 4H2O(g)����H =2��H1����H 2=-1453.28kJ��mol��1
��ԭ������������������������O2+4e-+2H2O=4OH-
ͭ�����ʵ���Ϊ0.1mol , �������ļ״�������Ϊ1.07g


 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣�
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
��1��S��N��O�ĵĵ�һ�������ɴ�С��˳��Ϊ ��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������ ���塣
��3����������������������ߣ����ڵĻ�����ȫ��ʳƷ��ȫԽ��ԽΪ��������ע����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е��ǨC19.5 �棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е���64.65 �棩����ȩ������Cԭ�Ӳ�ȡ �ӻ������ʽ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ�__________ ��
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu(CO)Cl��H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ ��
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl���������������Cu���� ����
��Cu(CO)Cl��H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ�������6������λ��������ͼ���ü�ͷ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
��1��S��N��O�ĵĵ�һ�������ɴ�С��˳��Ϊ ��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������ ���塣
��3����������������������ߣ����ڵĻ�����ȫ��ʳƷ��ȫԽ��ԽΪ��������ע����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е��ǨC19.5 �棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е���64.65 �棩����ȩ������Cԭ�Ӳ�ȡ �ӻ������ʽ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ�
__________ ��
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu(CO)Cl��H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ ��
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl���������������Cu���� ����
��Cu(CO)Cl��H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ������� ������λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣��״���CH3OH����һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϡ���֪
CH3OH(1)+ O2(g) = CO(g) + 2H2O(g) �� ��Ha= ��443.64 kJ��mol��1
2CO(g) + O2(g) = 2CO2(g) �� ��H b =��566.0 kJ��mol��1
��1����д��CH3OH(1)����������ȫȼ������CO2��H2O��g�����Ȼ�ѧ����ʽ��
��2���״���Ϊȼ�ϣ������ŵ���ȼ��ʱ�ŷŵ���Ⱦ���٣��Ӷ������ܻ�����Դ���ź�����ЧӦ�����⣬���ܸ��ƴ��������������ã�1���е��Ȼ�ѧ����ʽ���㣬��ȫȼ��20g�״������ɶ�����̼��ˮ����ʱ���ų�������Ϊ kJ �����ɵ�CO2�����״��������� L
��3��������Ա�½�������һ���ɼ״���������ǿ�����������Һ�������ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε磬�ݴ˻ش��������⣺
�״��� ����Ӧ���缫��ӦʽΪ ��
��4�����õ�ؿ�ʵ�ֵ�����ѧ��ת����ijͬѧ�����һ�ֵ�ⷨ��ȡFe(OH)2��ʵ��װ�ã�����ͼ��ʾ����ͨ�����Һ�в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ������˵������ȷ���� ������ţ�
A����Դ�е�aһ��Ϊ������bһ��Ϊ����
B��������NaCl��Һ��Ϊ���Һ
C��A��B���˶������������缫
D�����������ķ�Ӧ�ǣ�2H+ + 2e����H2��
��������Fe(OH)2������¶�ڿ����У�����ɫ�仯�� ����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ�������и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
��1��S��N��O�ĵĵ�һ�������ɴ�С��˳��Ϊ ��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������ ���塣
��3����������������������ߣ����ڵĻ�����ȫ��ʳƷ��ȫԽ��ԽΪ��������ע����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е��ǨC19.5 �棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е���64.65 �棩����ȩ������Cԭ�Ӳ�ȡ �ӻ������ʽ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ�
__________ ��
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu(CO)Cl��H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ ��
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl���������������Cu���� ����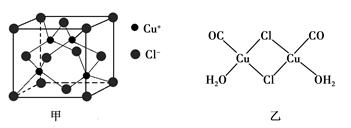
��Cu(CO)Cl��H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ������� ������λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ�����и�����Ӧ����ϰ�����ۣ���ѧ���� ���ͣ������
��13�֣�
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
��1��S��N��O�ĵĵ�һ�������ɴ�С��˳��Ϊ ��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������ ���塣
��3����������������������ߣ����ڵĻ�����ȫ��ʳƷ��ȫԽ��ԽΪ��������ע����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е��ǨC19.5 �棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е���64.65 �棩����ȩ������Cԭ�Ӳ�ȡ �ӻ������ʽ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ�__________ ��
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu(CO)Cl��H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ ��
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl���������������Cu���� ����
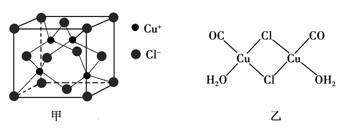
��Cu(CO)Cl��H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ�������6������λ��������ͼ���ü�ͷ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com