


 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ�鷽�� | ������ |
| A | �������� | ������ɫ���壬����������þ����Ԫ�� |
| B | ����NaOH ��Һ | ����ɫ���������������Ԫ�� |
| C | ���������������Һ���ټ�������NaOH��Һ | ������ɫ����������þԪ�� |
| D | ����KSCN ��Һ | ��Һ��Ѫ��ɫ��������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
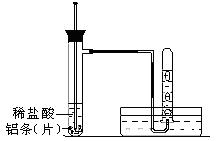
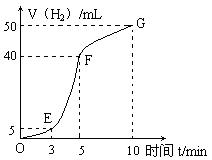
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
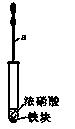
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | �������� | ʵ��Ŀ�� |
| A | | ̽�����ڸ�������е�������� |
| B | ����������ע������ˮ��û����������ֲ����Һ�� | |
| C | | ̽�������п�����ˮ��ʱ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
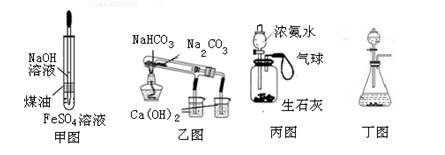
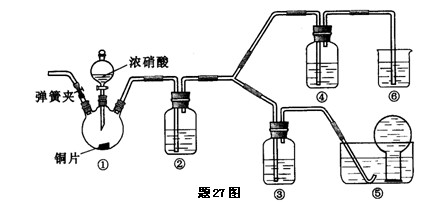
| A���ü�ͼװ���Ʊ����������������� |
| B������ͼװ����֤NaHCO3��Na2CO3�����ȶ��� |
| C���ñ�ͼװ�ÿ���ʵ��ʹ�������� |
| D���ö�ͼװ�ÿ�������ʵ������ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ��Ӧ�¶� /�� | �μӷ�Ӧ���� | ||||
| KMnO4 | H2C2O4 | MnSO4 | ||||
| V/mL | c/mol��L-1 | V/mL | c/mol��L-1 | m/g | ||
| A | 20 | 4 | 0.1 | 2 | 0.1 | 0 |
| B | 20 | 4 | 0.1 | 2 | 0.1 | 0.1 |
| C | 40 | 4 | 0.1 | 2 | 0.1 | 0 |
| D | 40 | 4 | 0.1 | 2 | 0.2 | 0.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ѧ������ļ��費һ���ܱ�ʵ��֤ʵ |
| B��ͬʱ�ı������������о���Ӧ���ʵı仯���ܸ���ó��йع��� |
| C����HF��HCl��HBr��HI���Ե�������ʵ���Ƴ�F��Cl��Br��I�ķǽ����Ե����Ĺ��� |
| D���ƶ��Ͳ��ն������ۻ�ѧ����Ĺ���ŵ������ѧ������־�Ż�ѧ�ѳ�Ϊ�������о�Ϊ����ѧ�ƣ�����Ҫ������ѧʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
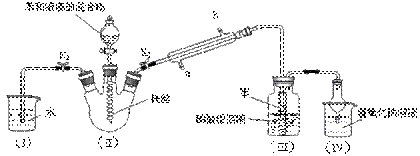
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com