
| 0.02mol |
| 0.1L |
| ||
| 0.02mol |
| 0.1L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HCl��������ˮ�� |
| B�������� |
| C������������ˮ |
| D��������������̿���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | ʵ������ |
| ��ȡ����ԭ��Һ������ϡ���ᣬ | ������ɫ���壬��������ʹ�����ʯ��ˮ����� |
| ��ȡ����ԭ��Һ����Ũ��������CuƬ��һ��Ũ�ȵ�H2SO4 | ����ɫ�����������������������ɺ���ɫ |
| ��ȡ����ԭ��Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ�����ϲ���Һ���ȼ���ϡ���ᣬ�ټ���AgNO3��Һ | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
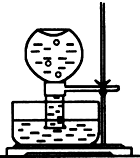 ��1����ͼ��ʾ������һƿ������ˮ������ˮ���У����չ����䵽ʢ����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���ǣ�������صķ�Ӧ����ʽ�ͼ�Ҫ����˵����
��1����ͼ��ʾ������һƿ������ˮ������ˮ���У����չ����䵽ʢ����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���ǣ�������صķ�Ӧ����ʽ�ͼ�Ҫ����˵�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
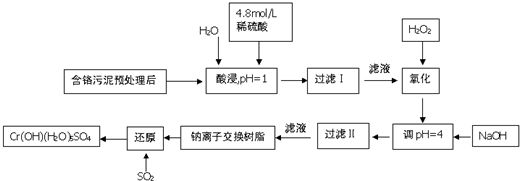
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Ca2+ | Cr3+ |
| ��ʼ����ʱ��pH | 1.9 | 7.0 | 9.6 | 4.2 | 9.7 | - |
| ������ȫʱ��pH | 3.2 | 9.0 | 11.1 | 8.0 | 11.7 | 9.0����9.0�ܽ⣩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���÷�ĩ��һ������Al��NO3��3��KOH��NaCl |
| B���÷�ĩ��һ������Al��NO3��3����KOH��������ȷ���Ƿ���NaCl |
| C���÷�ĩ��һ������NaCl��������ȷ���Ƿ���Al��NO3��3����KOH |
| D�����Ϲ��̲���ȷ������Һ�к����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� |
| a���ø���ྻ���ձ�ȡԼ10mLŨ���ᣬ���ȣ� |  |
| b����С���պ��ľ̿Ѹ�������ȵ�Ũ�����У� | ���ȵ�ľ̿���ȵ�Ũ����Ӵ��������ҷ�Ӧ��ͬʱ�д�������ɫ���������Һ����ľ̿Ѹ��ȼ�գ����������� |
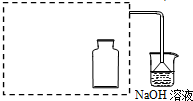
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��3.4mol/L |
| B��0.2mol/L |
| C��1.8mol/L |
| D��3.6mol/L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com