
ЁОЬтФПЁПЙ§ЬМЫсФЦ(Na2CO4)ЪЧвЛжжКмКУЕФЙЉбѕМСЃЌЦфгыЯЁбЮЫсЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2Na2CO4ЃЋ4HCl=4NaClЃЋ2CO2ЁќЃЋO2ЁќЃЋ2H2OЁЃЪаЪлЙ§ЬМЫсФЦвЛАуЖМКЌгаЬМЫсФЦЃЌЮЊВтЖЈФГЙ§ЬМЫсФЦбљЦЗ(жЛКЌNa2CO4КЭNa2CO3)ЕФДПЖШЃЌФГЛЏбЇаЫШЄаЁзщВЩгУвдЯТСНжжЗНАИЪЕЪЉЃК
ЗНАИвЛЃК![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
(1)ВйзїЂйКЭЂлЕФУћГЦЗжБ№ЮЊ______________________ЁЃ
(2)ЩЯЪіВйзїжаЃЌЪЙгУЕНВЃСЇАєЕФга _________________(ЬюВйзїађКХ)ЁЃ
(3)ЧыМђЪіВйзїЂлЕФВйзїЙ§ГЬЃК________________________________________________ЁЃ
ЗНАИЖўЃКАДЯТЭМЫљЪОАВзАКУЪЕбщзАжУЃЌQЮЊвЛЫмСЯЦјДќЃЌЫцвтШЁЪЪСПбљЦЗгкЦфжаЃЌДђПЊЗжвКТЉЖЗЛюШћЃЌНЋЯЁбЮЫсЕЮШыЦјДќжажСГфЗжЗДгІЁЃ

(4)ЮЊВтЖЈЗДгІЩњГЩЦјЬхЕФзмЬхЛ§ЃЌЕЮЯЁбЮЫсЧАБиаыЙиБе______(ЬюЁАK1ЁБЁАK2ЁБЛђЁАK3ЁБЃЌЯТЭЌ)ЃЌДђПЊ________ЁЃЕМЙмAЕФзїгУЪЧ_____________________________________________ЁЃ
(5)ЕБЩЯЪіЗДгІЭЃжЙКѓЃЌЪЙK1ЁЂK3ДІгкЙиБезДЬЌЃЌK2ДІгкДђПЊзДЬЌЃЌдйЛКЛКДђПЊKlЁЃ BжазАЕФЙЬЬхЪдМСЪЧ________________________ЁЃ
(6)ЪЕбщНсЪјЪБЃЌСПЭВЂёжагаx mLЫЎЃЌСПЭВЂђжаЪеМЏЕНСЫy mLЦјЬхЃЌдђбљЦЗжаЙ§ЬМЫсФЦЕФжЪСПЗжЪ§ЪЧ___________________ЁЃ
ЁОД№АИЁПГЦСПЁЂеєЗЂНсОЇ ЂкЂл МгШШеєЗЂжСеєЗЂУѓжаГіЯжДѓСПОЇЬхЪБЃЌЭЃжЙМгШШЃЌРћгУгрШШеєИЩеєЗЂУѓжаЕФЪЃгрЫЎЗж K1ЃЌK2 K3 ЦНКтЗжвКТЉЖЗФкКЭЗДгІЬхЯЕФкбЙЧПЃЌЪЙЯЁбЮЫсЫГРћЕЮЯТЃЌЭЌЪБЯћГ§ЕЮЯТЯЁбЮЫсЕФЬхЛ§ЖдЦјЬхЬхЛ§ЕФгАЯь МюЪЏЛв 122y/(53x-37y)
ЁОНтЮіЁП
ВтЖЈФГЙ§ЬМЫсФЦбљЦЗ(жЛКЌNa2CO4КЭNa2CO3)ЕФДПЖШЃЌбљЦЗГЦСПжЪСПЕУЕНбљЦЗжЪСПЮЊm1gЃЌМгШыЯЁбЮЫсШмНтКѓЙ§ТЫЕУЕНШмвКЮЊТШЛЏФЦШмвКЃЌеєЗЂХЈЫѕНсОЇЮіГіТШЛЏФЦЃЌГЦСПЕУЕНЙЬЬхТШЛЏФЦЕФжЪСПЮЊm2gЃЌИљОнЗНАИвЛСїГЬПЩХаЖЯГіВйзїЂйЮЊГЦСПЃЌВйзїЂкЮЊШмНтЃЌВйзїЂлЮЊеєЗЂНсОЇЁЃЗНАИЖўжаСПЭВЂёжаЫЎЕФЬхЛ§ЮЊВњЩњЕФCO2ЁЂO2ЕФЬхЛ§ЃЌСПЭВЂђжаЪеМЏЕНЕФЦјЬхЮЊO2ЃЌИљОнЗНАИЖўвРОнВтЖЈЕФбѕЦјЬхЛ§МЦЫуЮяжЪЕФСПЃЌНсКЯЛЏбЇЗНГЬЪНМЦЫуЙ§ЬМЫсФЦЮяжЪЕФСПМЦЫуЕУЕНжЪСПЗжЪ§ЁЃвдДЫЗжЮіНтД№ЁЃ
ИљОнЗНАИвЛСїГЬПЩХаЖЯГіВйзїЂйЮЊГЦСПЃЌВйзїЂкЮЊШмНтЃЌВйзїЂлЮЊеєЗЂНсОЇЁЃ
ЃЈ1ЃЉгЩЩЯЪіЗжЮіПЩжЊВйзїЂйЮЊГЦСПЃЌВйзїЂлЮЊеєЗЂНсОЇЃЌ
ЙЪД№АИЮЊЃКГЦСПЁЂеєЗЂНсОЇЃЛ
ЃЈ2ЃЉЩЯЪіВйзїжагУЕНВЃСЇАєЕФгаШмНтКЭеєЗЂНсОЇЃЌ
ЙЪД№АИЮЊЃКЂкЂлЃЛ
ЃЈ3ЃЉВйзїЂлЮЊеєЗЂНсОЇЃЌЦфОпЬхЙ§ГЬЮЊЃКМгШШеєЗЂжСеєЗЂУѓжаГіЯжДѓСПОЇЬхЪБЃЌЭЃжЙМгШШЃЌРћгУгрШШеєИЩеєЗЂУѓжаЕФЙЬЬхЃЌ
ЙЪД№АИЮЊЃКМгШШеєЗЂжСеєЗЂУѓжаГіЯжДѓСПОЇЬхЪБЃЌЭЃжЙМгШШЃЌРћгУгрШШеєИЩеєЗЂУѓжаЕФЪЃгрЫЎЗжЃЛ
ЃЈ4ЃЉЗДгІВњЩњЕФCO2ЁЂO2ЪЙЦјДќБфДѓЃЌНЋЙуПкЦПжаЦјЬхХХГіЃЌЫЎНјШыСПЭВЂёжаЃЌЫљвдСПЭВЂёжаЫЎЕФЬхЛ§МДЮЊВњЩњЕФCO2ЁЂO2ЕФЬхЛ§ЃЌЫљвдЕЮЯЁбЮЫсЧАБиаыЙиБеK1ЁЂK2ЃЌДђПЊK3ЁЃЕМЙмAЕФзїгУЪЧЦНКтЗжвКТЉЖЗФкКЭЗДгІЬхЯЕФкбЙЧПЃЌЪЙЯЁбЮЫсЫГРћЕЮЯТЃЌЭЌЪБЯћГ§ЕЮЯТЯЁбЮЫсЕФЬхЛ§ЖдЦјЬхЬхЛ§ЕФгАЯьЃЌ
ЙЪД№АИЮЊЃКK1ЃЌK2 ЃЛK3 ЃЛЦНКтЗжвКТЉЖЗФкКЭЗДгІЬхЯЕФкбЙЧПЃЌЪЙЯЁбЮЫсЫГРћЕЮЯТЃЌЭЌЪБЯћГ§ЕЮЯТЯЁбЮЫсЕФЬхЛ§ЖдЦјЬхЬхЛ§ЕФгАЯьЃЛ
ЃЈ5ЃЉBзАжУгУРДГ§ШЅЩњГЩЦјЬхжаЕФCO2ЃЌдђBжаЫљзАЙЬЬхЪдМСЮЊМюЪЏЛвЁЃЛКТ§ДђПЊK1ЪЧЮЊСЫШУЩњГЩЕФCO2ФмЙЛБЛBзАжУжаМюЪЏЛвГфЗжЮќЪеЃЌДгЖјЪЙСПЭВжаЪеМЏЕННЯДПЕФO2ЃЌ
ЙЪД№АИЮЊЃКМюЪЏЛвЃЛ
ЃЈ6ЃЉвРЬтвтПЩжЊЃЌСПЭВЂђжаЪеМЏЕНЕФЦјЬхЮЊO2ЃЌСПЭВЂёжаЫЎЕФЬхЛ§ЕШгкЗДгІВњЩњЕФCO2гыO2ЕФзмЬхЛ§ЃЌдђПЩНјааШчЯТМЦЫуЃК
n(Na2CO4)=2 n(O2)=![]() mmolЃЌn(CO2)=
mmolЃЌn(CO2)=![]() mmolЃЌNa2CO4гыбЮЫсЗДгІЩњГЩCO2ЕФЮяжЪЕФСПЮЊn(CO2)1=
mmolЃЌNa2CO4гыбЮЫсЗДгІЩњГЩCO2ЕФЮяжЪЕФСПЮЊn(CO2)1=![]() mmolЃЌгжNa2CO3гыбЮЫсЗДгІЩњГЩCO2ЕФМЦСПЙиЯЕЮЊNa2CO3~CO2ЃЌдђn(Na2CO3)= n(CO2)2=
mmolЃЌгжNa2CO3гыбЮЫсЗДгІЩњГЩCO2ЕФМЦСПЙиЯЕЮЊNa2CO3~CO2ЃЌдђn(Na2CO3)= n(CO2)2=![]() mmolЃЌ
mmolЃЌ
ЫљвдNa2CO4ЕФжЪСПЗжЪ§w( Na2CO4)%=![]() 100%=122y/(53x-37y)ЁЃ
100%=122y/(53x-37y)ЁЃ
ЙЪД№АИЮЊЃК122y/(53x-37y)ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙЄвЕЩЯРћгУСђЬњПѓЩедќЃЈжївЊГЩЗжЮЊFe2O3ЁЂFeOЁЂSiO2ЕШЃЉЮЊдСЯжЦБИИпЕЕбеСЯЬњКь(Fe2O3)ЃЌОпЬхЩњВњСїГЬШчЭМЃК
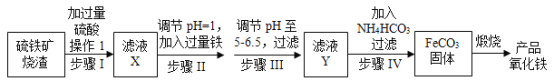
ЪдЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉТЫвКXжаКЌгаЕФН№ЪєбєРызгЪЧ___ЃЈЬюРызгЗћКХЃЉЁЃ
ЃЈ2ЃЉВНжшЂђжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_____ЁЃ
ЃЈ3ЃЉВНжшЂѓжазюКУПЩбЁгУ___ЃЈЬюзжФИЃЉЕїНкШмвКЕФpHЁЃ
AЃЎЯЁЯѕЫс BЃЎАБЫЎ CЃЎЧтбѕЛЏФЦШмвК DЃЎИпУЬЫсМиШмвК
ЃЈ4ЃЉВНжшЂєжаЃЌFeCO3ГСЕэЭъШЋКѓЃЌШмвКжаКЌгаЩйСПFe2ЃЋЃЌМьбщFe2ЃЋЕФЗНЗЈЪЧ___ЁЃ
ЃЈ5ЃЉВНжшЂєЕФЗДгІЮТЖШвЛАуашПижЦдк35ЁцвдЯТЃЌЦфФПЕФЪЧ______ЁЃ
ЃЈ6ЃЉдкПеЦјжаьбЩеFeCO3ЩњГЩВњЦЗбѕЛЏЬњЕФЛЏбЇЗНГЬЪНЮЊ_____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкСНЗнЯрЭЌЕФBa(OH)2ШмвКжаЃЌЗжБ№ЕЮШыЮяжЪЕФСПХЈЖШЯрЕШЕФH2SO4ЁЂNaHSO4ШмвКЃЌЦфЕМЕчФмСІЫцЕЮШыШмвКЬхЛ§БфЛЏЕФЧњЯпШчЭМЫљЪОЁЃЯТСаЗжЮіе§ШЗЕФЪЧ

A.bЁњdЗДгІЕФРызгЗНГЬЪНЮЊЃКH++OH-=H2O
B.oЁњaЗДгІЕФРызгЗНГЬЪНЮЊЃКBa2++OH-+H++SO42-=BaSO4Ё§+H2O
C.cЕуЕМЕчФмСІЯрЭЌЃЌЫљвдСНШмвКжаКЌгаЯрЭЌСПЕФOHЈC
D.aЁЂbСНЕуBa2+ОљГСЕэЭъШЋЃЌЫљвдЖдгІЕФШмвКОљЯджаад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкдЊЫиWЁЂXЁЂYЁЂZЕФдзгађЪ§вРДЮдіМгЁЃmЁЂpЁЂrЪЧгЩетаЉдЊЫизщГЩЕФЖўдЊЛЏКЯЮяЃЌqЪЧYЕФЕЅжЪЧвЮЊЕЛЦЩЋЙЬЬхЃЌnЪЧдЊЫиZЕФЕЅжЪЃЌГЃЮТЯТЪЧЛЦТЬЩЋЕФЦјЬхЃЌ0.01molЁЄLЈC1rШмвКЕФpHЮЊ2ЃЌsЭЈГЃЪЧФбШмгкЫЎЕФЛьКЯЮяЁЃЩЯЪіЮяжЪЕФзЊЛЏЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈвЛЖЈе§ШЗЕФЪЧ
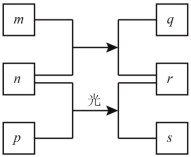
A.дзгАыОЖЕФДѓаЁЃКW<X<Y<Z
B.дЊЫиЕФЗЧН№ЪєадЃКX>Y
C.МђЕЅЧтЛЏЮяЕФЮШЖЈадЃКZ>Y>X
D.бѕЛЏЮяЕФЖдгІЫЎЛЏЮяЕФЫсадЃКZ>Y
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДПОЛИЩдяЕФТШЦјгыШлШкН№ЪєЮ§ЗДгІПЩжЦБИ SnCl4ЃЌФГЛЏбЇаЁзщЕФЭЌбЇЩшМЦСЫШчЯТЪЕбщзАжУНјаажЦБИЁЃ

вбжЊЃКЂйН№ЪєЮ§ШлЕуЮЊ 231ЁцЃЌЛЏбЇЛюЦУадгыЬњЯрЫЦЃЛЂкSnCl4 ЕФЗаЕуЮЊ 114ЁцЃЛЂлSnCl4 взгыЫЎЗДгІЁЃ
ЧыИљОнЩЯЭМзАжУЛиД№:
ЃЈ1ЃЉзАжУЂєжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________________________ЁЃ
ЃЈ2ЃЉЪдЙмIIжаЕФЪдМСЪЧ___________________ЃЌзАжУ V ЕФзїгУЪЧ______________ЁЃ
ЃЈ3ЃЉбbжУЂізюКУбЁгУЯТСазАжУжаЕФ______________________(ЬюБъКХ)ЁЃ

ЃЈ4ЃЉЪЕбщНсЪјКѓЃЌгћЛиЪеРћгУзАжУЂёжаЮДЗДгІЭъЕФ MnO2ЃЌашвЊЕФЗжРыЗНЗЈ___________ЁЃ
ЃЈ5ЃЉЮЊСЫЫГРћЭъГЩЪЕбщЃЌЕуШМОЦОЋЕЦЕФе§ШЗВйзїЪЧ___________________(ЬюзжФИ)ЁЃ
AЃЎЯШЕуШМ I ДІОЦОЋЕЦЃЌКѓЕуШМЂєДІОЦОЋЕЦ
BЃЎЯШЕуШМЂєДІОЦОЋЕЦЃЌКѓЕуШМ I ДІОЦОЋЕЦ
CЃЎЭЌЪБЕуШМ IЁЂЂєСНДІОЦОЋЕЦ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПТЬЗЏЪЧКЌгавЛЖЈСПНсОЇЫЎЕФСђЫсбЧЬњЃЌдкЙЄХЉвЕЩњВњжаОпгаживЊЕФгУЭОЁЃФГЛЏбЇаЫШЄаЁзщЖдТЬЗЏЕФвЛаЉаджЪНјааЬНОПЁЃЛиД№ЯТСаЮЪЬтЃК
(1)ШчКЮгУЪЕбщжЄУїТЬЗЏжаЕФЬњЪЧЖўМлЬњЖјВЛЪЧШ§МлЬњ_______ЁЃ
(2)ЮЊВтЖЈТЬЗЏжаНсОЇЫЎКЌСПЃЌНЋЪЏгЂВЃСЇЙмЃЈДјСНЖЫПЊЙиK1КЭK2ЃЉЃЈЩшЮЊзАжУAЃЉГЦжиЃЌМЧЮЊm1gЁЃНЋбљЦЗзАШыЪЏгЂВЃСЇЙмжаЃЌдйДЮНЋзАжУAГЦжиЃЌМЧЮЊm2gЁЃАДШчЭМСЌНгКУзАжУНјааЪЕбщЁЃ
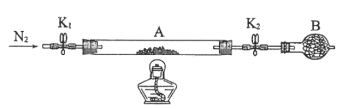
ЂйвЧЦїBЕФУћГЦЪЧ________ЁЃ
ЂкНЋЯТСаЪЕбщВйзїВНжше§ШЗХХађ_______ЃЈЬюБъКХЃЉЃЛжиИДЩЯЪіВйзїВНжшЃЌжБжСAКужиЃЌМЧЮЊm3gЁЃ
a.ЕуШМОЦОЋЕЦЃЌМгШШ b.ДђПЊK1КЭK2ЃЌЛКЛКЭЈШыN2 c.ЙиБеK1КЭK2
d.ЯЈУ№ОЦОЋЕЦ e.ГЦСПA f.РфШДЕНЪвЮТ
ЂлИљОнЪЕбщМЧТМЃЌМЦЫуТЬЗЏЛЏбЇЪНжаНсОЇЫЎЪ§ФПx=_______(СаЪНБэЪО)ЁЃШєЪЕбщЪБАДaЁЂbДЮађВйзїЃЌдђЪЙx_______ЃЈЬюЁАЦЋДѓЁБЁАЦЋаЁЁБЛђЁАЮогАЯьЁБЃЉЁЃ
(3)ЮЊЬНОПСђЫсбЧЬњЕФЗжНтВњЮяЃЌНЋ(2)жавбКужиЕФзАжУAНгШыШчЭМЫљЪОЕФзАжУжаЃЌДђПЊK1КЭK2ЃЌЛКЛКЭЈШыN2ЃЌМгШШЁЃЪЕбщКѓЗДгІЙмжаВаСєЙЬЬхЮЊКьЩЋЗлФЉЁЃ
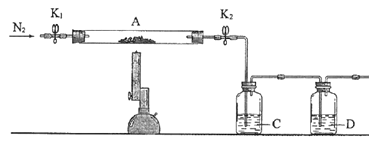
ЂйCжаЕФШмвКЮЊBaCl2ЃЌЦфзїгУЪЧ______ЁЃDШмвКЮЊ______ЃЌDжаПЩЙлВьЕНЕФЯжЯѓЮЊ_______ЁЃ
ЂкаДГіСђЫсбЧЬњИпЮТЗжНтЗДгІЕФЛЏбЇЗНГЬЪН________ЁЃ
ЂлгаЭЌбЇШЯЮЊИУЪЕбщзАжУДцдквЛЖЈШБЯнЃЌЧыФуЬИЬИЭъЩЦИУЪдбщзАжУЕФДыЪЉ____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪвгУЯТЪізАжУжЦШЁТШЦјЃЌВЂгУТШЦјНјааЯТСаЪЕбщЁЃПДЭМЛиД№ЯТСаЮЪЬтЃК
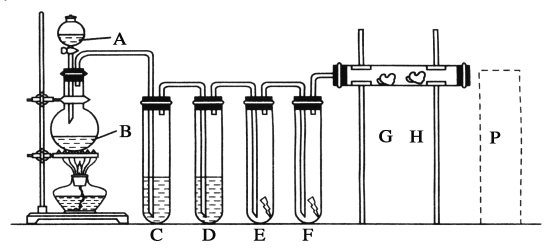
(1)AЁЂBСНвЧЦїЕФУћГЦЃКA________ЃЌB________ЁЃ
(2)ЯДЦјзАжУCЪЧЮЊСЫГ§ШЅCl2жаЕФHClЦјЬхЃЌDЪЧЮЊСЫИЩдяCl2ЃЌдђCЁЂDжагІЗжБ№ЗХШыЯТСаШмвКжаЕФC______________ЃЛD______________ЁЃ
ЂйNaOHШмвКЁЁЂкБЅКЭЪГбЮЫЎЁЁЂлAgNO3ШмвКЁЁЂмХЈH2SO4
(3)EжаЮЊКьЩЋИЩВМЬѕЃЌFжаЮЊКьЩЋЪЊВМЬѕЃЌПЩЙлВьЕНЕФЯжЯѓЪЧ______________________________ЁЃ
(4)GЪЧНўгаЕэЗлKIШмвКЕФУоЛЈЧђЃЌGДІЯжЯѓЪЧУоЛЈЧђБэУцБфГЩ____________ЁЃЗДгІЕФРызгЗНГЬЪНЪЧ______________________ЃЌHЪЧНўгаNaBrШмвКЕФУоЛЈЧђЃЌHДІЯжЯѓЪЧУоЛЈЧђБэУцБфГЩ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдк2010ФъЮТИчЛЊЖЌМОАТдЫЛсЩЯЃЌгаИіБ№дЫЖЏдБвђЗўгУаЫЗмМСБЛШЁЯћВЮШќЕФзЪИёЁЃЯТЭМЪЧМьВтГіаЫЗмМСЕФФГжжЭЌЯЕЮяXЕФНсЙЙЃЌЙигкXЕФЫЕЗЈе§ШЗЕФЪЧ( )
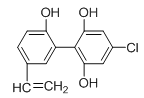
A. XЗжзгжаВЛПЩФмЫљгадзгЖМдкЭЌвЛЦНУцЩЯ
B. X гіЕНFeCl3ШмвКЪБЯдзЯЩЋЃЌЖјЧвФмЪЙфхЕФЫФТШЛЏЬМШмвКЭЪЩЋ
C. 1 mol X гызуСПЕФХЈфхЫЎЗДгІЃЌзюЖрЯћКФ5 mol Br2
D. 1 mol XдквЛЖЈЬѕМўЯТгызуСПЕФЧтЦјЗДгІЃЌзюЖрЯћКФ1 mol H2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдзгађЪ§аЁгк36ЕФXЁЂYЁЂZЁЂRЁЂWЮхжждЊЫиЃЌЦфжаXЪЧжмЦкБэжадзгАыОЖзюаЁЕФдЊЫиЃЌYЪЧаЮГЩЛЏКЯЮяжжРрзюЖрЕФдЊЫиЃЌZдзгЛљЬЌЪБ2pдзгЙьЕРЩЯга3ИіЮДГЩЖдЕФЕчзгЃЌRЕЅжЪеМПеЦјЬхЛ§ЕФ![]() ЃЛWЕФдзгађЪ§ЮЊ29ЁЃЛиД№ЯТСаЮЪЬт:
ЃЛWЕФдзгађЪ§ЮЊ29ЁЃЛиД№ЯТСаЮЪЬт:
ЃЈ1ЃЉY2X4ЗжзгжаYдзгЙьЕРЕФдгЛЏРраЭЮЊ________ЃЌ1mol Z2X4КЌгаІвМќЕФЪ§ФПЮЊ ________ЁЃ
ЃЈ2ЃЉЛЏКЯЮяZX3гыЛЏКЯЮяX2RЕФVSEPRЙЙаЭЯрЭЌЃЌЕЋСЂЬхЙЙаЭВЛЭЌЃЌZX3ЕФСЂЬхЙЙаЭЮЊ ________ЃЌСНжжЛЏКЯЮяЗжзгжаЛЏбЇМќЕФМќНЧНЯаЁЕФЪЧ________ЃЈгУЗжзгЪНБэЪОЃЌЯТЭЌ)ЁЃ
ЃЈ3ЃЉгыRЭЌжїзхЕФШ§жжЗЧН№ЪєдЊЫигыXПЩаЮГЩНсЙЙЯрЫЦЕФШ§жжЮяжЪЃЌЪдЭЦВтШ§епЕФЮШЖЈадгЩДѓЕНаЁЕФЫГађ________ЃЌРэгЩЪЧ ________ЃЛШ§епЕФЗаЕугЩИпЕНЕЭЕФЫГађЪЧ ________ЃЌНтЪЭдвђ________ЁЃ
ЃЈ4ЃЉдЊЫиYЕФвЛжжбѕЛЏЮягыдЊЫиZЕФЕЅжЪЛЅЮЊЕШЕчзгЬхЃЌдЊЫиYЕФетжжбѕЛЏЮяЕФЗжзгЪНЪЧ________ЁЃ
ЃЈ5ЃЉWдЊЫига________ИідЫЖЏзДЬЌВЛЭЌЕФЕчзгЃЌЦфЛљЬЌдзгЕФМлЕчзгХХВМЪНЮЊ________ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com