
��11�֣��о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ�Ŀ��⡣
��1��CO�����ںϳɼ״�����ѹǿΪ0.1Mpa�����£������ΪbL���ܱ������г���amolCO��2amolH2���ڴ��������ºϳɼ״���
CO��g��+2H2��g��CH3OH��g��ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��
��i���÷�Ӧ����_____________��Ӧ��������ȡ����ȡ�����
��ii��100��ʱ���÷�Ӧ��ƽ�ⳣ����K=_____________������a��b�Ĵ���ʽ��ʾ������һ�����淴Ӧ��ƽ�ⳣ��Kֵ�ܴԴ˷�Ӧ��˵����ȷ���ǣ�_________________����ţ�
�÷�Ӧʹ�ô������岻��
�÷�Ӧ�������ںܶ�ʱ������ɣ�
�÷�Ӧ�ﵽƽ��ʱ������һ�ַ�Ӧ��ٷֺ�����С��
�÷�Ӧһ���Ƿ��ȷ�Ӧ��
��iii�����¶Ⱥ��ݻ����������£�����ƽ����ϵ�г���amolCO��2amolH2���ﵽƽ��ʱCOת����________������������䡱��С������ͬ��ƽ�ⳣ����________��
��iv����ij�¶��£���һ�ݻ�������ܱ������г���2.5molCO��7.5molH2����Ӧ����CH3OH��g�����ﵽƽ��ʱ��COת����Ϊ90%����ʱ������ѹǿΪ��ʼʱ��ѹǿ__________����
��2��ij�¶������£�����CO2��g����H2��g���������1��4��ϣ����ʵ�ѹǿ�ʹ��������¿��Ƶü��飬��֪��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3KJ/mol
H2��g��+1/2O2��g��= H2O��l�� ��H=-285.8KJ/mol
��CO2��g����H2��g����Ӧ����Һ̬ˮ���Ȼ�ѧ����ʽΪ��__________________________________________________________________________��
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��������ģ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ���⣮CO�����ںϳɼ״�����ѹǿΪ0.1MPa�����£������Ϊb L���ܱ������г���a mol CO��2a mol H2���ڴ��������ºϳɼ״���CO��g��+2H2��g��?CH3OH��g����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
��2012?��������ģ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ���⣮CO�����ںϳɼ״�����ѹǿΪ0.1MPa�����£������Ϊb L���ܱ������г���a mol CO��2a mol H2���ڴ��������ºϳɼ״���CO��g��+2H2��g��?CH3OH��g����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�ϲ��и������в��Ի�ѧ�Ծ� ���ͣ������
��11�֣��о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ�Ŀ��⡣
��1��CO�����ںϳɼ״�����ѹǿΪ0.1Mpa�����£������ΪbL���ܱ������г���amolCO��2amolH2���ڴ��������ºϳɼ״���
CO��g��+2H2��g�� CH3OH��g��ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��
CH3OH��g��ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��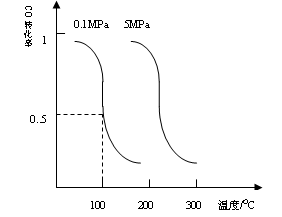
��i���÷�Ӧ����_____________��Ӧ��������ȡ����ȡ�����
��ii��100��ʱ���÷�Ӧ��ƽ�ⳣ����K=_____________������a��b�Ĵ���ʽ��ʾ����
��һ�����淴Ӧ��ƽ�ⳣ��Kֵ�ܴԴ˷�Ӧ��˵����ȷ���ǣ�_________________����ţ�
| A���÷�Ӧʹ�ô������岻�� |
| B���÷�Ӧ�������ںܶ�ʱ������ɣ� |
| C���÷�Ӧ�ﵽƽ��ʱ������һ�ַ�Ӧ��ٷֺ�����С�� |
| D���÷�Ӧһ���Ƿ��ȷ�Ӧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ׯһģ ���ͣ��ʴ���
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(08��ׯ����)ȼ�Ϻ���Դ�ǻ�ѧ֪ʶ�����������ϵ��Ϊ���е����ݡ�����Ҫ��ע������Դ�ĺ������ã������о�����������Դ��
��1������ԴӦ�þ���ԭ״���á�ȼ��ʱ�������������Ҳ�����Ⱦ�����ص㡣��ú̿��ʯ�͡�ú���������У�ǰ;��������Դ�� ��
��2�����������ҹ�ú���¹ʴ����������˹��ը���¡���˹�к��м����һ����̼�����壬��������˹Ũ�ȴﵽһ����Χʱ������ȼ�ձ�ը��Ϊ�������ѵķ���Ӧ��ȡ����ʵ���еĴ�ʩ�� ������ţ�
�ټ�ǿ��ȫ�������ž�����Դ �ڽ�����˹������Ż��
�����ͨ������ �ܽ����е�������ȥ
��3��Ϊ�����ú����ЧӦ��ͬʱ����ȼ��ʱ�Ļ�����Ⱦ������úת��Ϊˮú�������ǽ�úת��Ϊ�ྻȼ�ϵķ���֮һ��ˮú������Ҫ�ɷ���һ����̼��������������ú̿��ˮ������Ӧ�Ƶã���֪C��ʯī����CO��H2ȼ�յ��Ȼ�ѧ����ʽΪ��
C��s��ʯī��+O2��g�� CO2��g����H1=��393.5kJ?mol-1
H2��g��+![]() O2��g��
O2��g�� H2O��g����H2=��241.8kJ?mol-1
CO��g��+O![]() 2��g��
2��g�� CO2��g����H3=��283.0kJ?mol-1
H2��g��+![]() O2��g��
O2��g�� H2O��1����H4=��285.8kJ?mol-1
��ش��������⣺
�ٸ��������ṩ���Ȼ�ѧ����ʽ���㣬36gˮ��Һ̬�����̬�������仯�� ��
��д��C��s��ʯī����ˮ������Ӧ���Ȼ�ѧ����ʽ ��
�۱�����Һ��ʯ��������Ҫ�ɷ�֮һ������ȼ�յ��Ȼ�ѧ����ʽΪ��
C3H8��g��+5O2��g�� 3CO2��g��+4H2O��g����H=��2220.0kJ?mol-1
��ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ������������֮��Ϊ___����ͬ�����������ͱ�����ȫȼ��������̬����ʱ������������֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о��Ϳ���CO2 ��CO�Ĵ��������ǻ�����������Դ����
��˫Ӯ�Ŀ��⡣
I.CO�����ںϳɼ״�����ѹǿΪ0.1Mpa�����£������ΪbL����
�������г���amolCO��2amolH2���ڴ��������ºϳɼ״�;
![]() ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ:
ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ:
 (1)100��ʱ���÷�Ӧ��ƽ�ⳣ��:
(1)100��ʱ���÷�Ӧ��ƽ�ⳣ��:
K= ��(��a��b�Ĵ���ʽ��
ʾ)����һ�����淴Ӧ��ƽ�ⳣ��Kֵ
�� �Դ˷�Ӧ��˵����ȷ����:
(�����)
A.�÷�Ӧʹ�ô������岻��;
B.�÷�Ӧ�������ںܶ�ʱ�������;
C. �÷�Ӧ�ﵽƽ��ʱ������һ�ַ�Ӧ��
�ٷֺ�����С��
D.��Ӧ���ת����һ����:
(2)���¶Ⱥ��ݻ����������£�����ƽ����ϵ�г���amolCO��2amolH2���ﵽƽ��ʱ
COת���� (����������䡱��С������ͬ)ƽ�ⳣ���� ��
(3)��ij�¶��£���һ�ݻ�������ܱ������г���2.5molCO��7.5molH2����Ӧ����CH3OH
(g)���ﵽƽ��ʱ��COת����Ϊ90%����ʱ������ѹǿΪ��ʼʱ��ѹǿ ����
11.ij�¶������£�����CO2(g)��H2��g���������1:4��ϣ����ʵ�ѹǿ�ʹ���
�����¿��Ƶü��飬��֪��
![]()
��![]() ��
��![]() ��Ӧ����Һ̬ˮ���Ȼ�ѧ����ʽΪ:
��Ӧ����Һ̬ˮ���Ȼ�ѧ����ʽΪ:
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com