
”¾ĢāÄæ”æĪļÖŹŌŚĖ®ÖŠæÉÄÜ“ęŌŚµēĄėĘ½ŗā”¢ŃĪµÄĖ®½āĘ½ŗāŗĶ³ĮµķČܽāĘ½ŗā£¬ĖüĆĒ¶¼æÉæ“×÷»ÆŃ§Ę½ŗā”£Ēėøł¾ŻĖłŃ§ÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AlCl3ČÜŅŗ³Ź______ŠŌ(Ģī”°Ėį”±”°ÖŠ”±”°¼ī”±)ŌŅņŹĒ_________________________(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)£»Čō°ŃAlCl3ČÜŅŗÕōøÉ£¬×ĘÉÕ£¬×īŗóÖ÷ŅŖµĆµ½¹ĢĢå²śĪļŹĒ________£»Čō½«AlCl3ČÜŅŗŗĶNaHCO3ČÜŅŗ»ģŗĻ£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________________”£
(2)½«1L0.2 mol”¤L£1HAČÜŅŗÓė1L0.1 mol”¤L£1NaOHČÜŅŗµČĢå»ż»ģŗĻ(»ģŗĻŗóČÜŅŗĢå»ż±ä»ÆŗöĀŌ²»¼Ę)£¬²āµĆ»ģŗĻČÜŅŗÖŠc(Na£«)>c(A£)£¬Ōņ£ŗ
¢Ł»ģŗĻČÜŅŗÖŠ£¬c(A£)________c(HA)(Ģī”°>”±”°<”±»ņ”°£½”±£¬ĻĀĶ¬)”£
¢Ś»ģŗĻČÜŅŗÖŠ£¬c(HA)£«c(A£)________0.1 mol”¤L£1”£
(3)³£ĪĀĻĀ£¬ČōNaOHČÜŅŗÖŠc(OH”Ŗ)ÓėNH4ClČÜŅŗÖŠc(H+)ĻąĶ¬£¬ĻÖ½«NaOHČÜŅŗŗĶNH4ClČÜŅŗ·Ö±šĻ”ŹĶ10±¶£¬Ļ”ŹĶŗóNaOHČÜŅŗŗĶNH4CIČÜŅŗµÄpH·Ö±šÓĆpH1ŗĶpH2±ķŹ¾”£ŌņpH1 +pH2________(Ģī”°>”±”°<”±»ņ”°£½”±)14”£
(4)pHĻąĶ¬µÄ¢ŁCH3COONa”¢¢ŚNaHCO3”¢¢ŪNaClOČżÖÖČÜŅŗµÄc(Na£«)£ŗ___________________”£
”¾“š°ø”æĖį Al3£«£«3H2O![]() Al(OH)3£«3H£« Al2O3 Al3£«£«3HCO3££½Al(OH)3”ż£«3CO2”ü £¼ £½ £¼ ¢Ł£¾¢Ś£¾¢Ū
Al(OH)3£«3H£« Al2O3 Al3£«£«3HCO3££½Al(OH)3”ż£«3CO2”ü £¼ £½ £¼ ¢Ł£¾¢Ś£¾¢Ū
”¾½āĪö”æ
£Ø1£©øł¾ŻĀČ»ÆĀĮĖ®½āŅŌ¼°Ķā½ēĢõ¼ž¶ŌĖ®½āĘ½ŗāµÄÓ°Ļģ·ÖĪö£»øł¾Ż·“Ó¦ĪļŗĶÉś³ÉĪļŹéŠ“·½³ĢŹ½£»
£Ø2£©øł¾ŻµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗć·ÖĪöÅŠ¶Ļ£»
£Ø3£©øł¾ŻĻ”ŹĶ¹ż³ĢÖŠ¶ŌĖ®½āĘ½ŗāµÄÓ°Ļģ·ÖĪöÅŠ¶Ļ£»
£Ø4£©øł¾ŻĖįŌ½Čõ£¬ĻąÓ¦µÄĖįøłŌ½ČŻŅ×Ė®½ā·ÖĪö”£
£Ø1£©ĀČ»ÆĀĮČÜŅŗÖŠĀĮĄė×ÓĖ®½ā£¬ČÜŅŗĻŌĖįŠŌ£¬Ė®½ā·½³ĢŹ½ĪŖAl3£«£«3H2O![]() Al(OH)3£«3H£«£»Ė®½āĪüČČ£¬¼ÓČČ“Ł½ųĖ®½ā£¬Éś³ÉµÄHClŅ×»Ó·¢£¬×īŗóÕōøɵƵ½ĒāŃõ»ÆĀĮ£¬×ĘÉÕŹ±ĒāŃõ»ÆĀĮ·Ö½āÉś³ÉŃõ»ÆĀĮŗĶĖ®£¬ĖłŅŌ×īŗóÖ÷ŅŖµĆµ½¹ĢĢå²śĪļŹĒAl2O3£»Čō½«AlCl3ČÜŅŗŗĶNaHCO3ČÜŅŗ»ģŗĻ¶žÕßĖ®½āĻą»„“Ł½ųÉś³ÉĒāŃõ»ÆĀĮŗĶ¶žŃõ»ÆĢ¼£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl3£«£«3HCO3££½Al(OH)3”ż£«3CO2”ü”£
Al(OH)3£«3H£«£»Ė®½āĪüČČ£¬¼ÓČČ“Ł½ųĖ®½ā£¬Éś³ÉµÄHClŅ×»Ó·¢£¬×īŗóÕōøɵƵ½ĒāŃõ»ÆĀĮ£¬×ĘÉÕŹ±ĒāŃõ»ÆĀĮ·Ö½āÉś³ÉŃõ»ÆĀĮŗĶĖ®£¬ĖłŅŌ×īŗóÖ÷ŅŖµĆµ½¹ĢĢå²śĪļŹĒAl2O3£»Čō½«AlCl3ČÜŅŗŗĶNaHCO3ČÜŅŗ»ģŗĻ¶žÕßĖ®½āĻą»„“Ł½ųÉś³ÉĒāŃõ»ÆĀĮŗĶ¶žŃõ»ÆĢ¼£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl3£«£«3HCO3££½Al(OH)3”ż£«3CO2”ü”£
£Ø2£©¢Ł½«1L0.2 mol”¤L£1HAČÜŅŗÓė1L0.1 mol”¤L£1NaOHČÜŅŗµČĢå»ż»ģŗĻ(»ģŗĻŗóČÜŅŗĢå»ż±ä»ÆŗöĀŌ²»¼Ę)£¬ĖłµĆČÜŅŗÖŠČÜÖŹŹĒµČÅØ¶ČµÄHAŗĶNaAµÄ»ģŗĻČÜŅŗ£¬²āµĆ»ģŗĻČÜŅŗÖŠc(Na£«)£¾c(A£)£¬Ōņøł¾ŻµēŗÉŹŲŗćc(Na£«)+c(H£«)£½c(A£)+c(OH”Ŗ)æÉÖŖc(H£«)£¼c(OH”Ŗ)£¬¼“ČÜŅŗĻŌ¼īŠŌ£¬ÕāĖµĆ÷Ė®½ā³Ģ¶Č“óÓŚµēĄė³Ģ¶Č£¬ĖłŅŌ»ģŗĻČÜŅŗÖŠ£¬c(A£)£¼c(HA)”£
¢ŚĖłµĆČÜŅŗÖŠČÜÖŹŹĒµČÅØ¶ČµÄHAŗĶNaAµÄ»ģŗĻČÜŅŗ£¬ÅØ¶Č¾łŹĒ0.05mol/L£¬Ōņøł¾ŻĪļĮĻŹŲŗćæÉÖŖ»ģŗĻČÜŅŗÖŠ£¬c(HA)£«c(A£)£½0.1 mol”¤L£1”£
£Ø3£©ÉčĻ”ŹĶĒ°NaOHČÜŅŗŗĶNH4ClČÜŅŗµÄpH·Ö±šÓĆpH3ŗĶpH4±ķŹ¾£¬½«NaOHČÜŅŗĻ”ŹĶ10±¶£¬ÓÉÓŚĒāŃõ»ÆÄĘŹĒŅ»ŌŖĒæ¼ī£¬ŌņpH1£½pH3£1£»ĀČ»Æļ§ŹĒĒæĖįČõ¼īŃĪ£¬ļ§øłĖ®½ā£¬Ļ”ŹĶ“Ł½ųļ§øłĖ®½ā£¬ŌņĻ”ŹĶ10±¶ŗópH2£¼pH4+1£¬ÓÉÓŚNaOHČÜŅŗÖŠc(OH”Ŗ)ÓėNH4ClČÜŅŗÖŠc(H+)ĻąĶ¬£¬¼“pH3+pH4£½14£¬ĖłŅŌpH1 +pH2£¼pH3£1+pH4+1£½14”£
£Ø4£©ČõĖįøłĄė×ÓµÄĖ®½ā³Ģ¶ČŌ½“ó£¬ĻąĶ¬pHµÄÄĘŃĪČÜŅŗµÄÅضČŌ½Š”£¬ÄĘĄė×ÓÅضČŌ½Š”£¬ÓÉÓŚĖ®½ā³Ģ¶Č¢Ł£¼¢Ś£¼¢Ū£¬ŌņpHĻąĶ¬µÄ¢ŁCH3COONa”¢¢ŚNaHCO3”¢¢ŪNaClOČżÖÖČÜŅŗµÄc(Na£«)ĪŖ¢Ł£¾¢Ś£¾¢Ū”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÄ³Ń§ÉśÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗŹ±£¬Ń”Ōń¼×»ł³Č×÷ÖøŹ¾¼Į”£ĒėĢīŠ“ĻĀĮŠæÕ°×”£
(1)ÓƱź×¼µÄŃĪĖįµĪ¶Ø“ż²āµÄNaOHČÜŅŗŹ±£¬×óŹÖĪÕĖįŹ½µĪ¶Ø¹ÜµÄ»īČū£¬ÓŅŹÖŅ”¶Æ׶ŠĪĘ棬ŃŪ¾¦×¢ŹÓ_______£¬Ö±µ½Ņņ¼ÓČėŅ»µĪŃĪĖįŗó£¬ČÜŅŗÓÉ________É«±äĪŖ________É«£¬²¢________ĪŖÖ¹”£
(2)ĻĀĮŠ²Ł×÷ÖŠæÉÄÜŹ¹Ėł²āNaOHČÜŅŗµÄÅØ¶ČŹżÖµĘ«µĶµÄŹĒ__________”£
A£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČóĻ“¾ĶÖ±½Ó×¢Čė±ź×¼ŃĪĖį
B£®µĪ¶ØĒ°Ź¢·ÅNaOHČÜŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊøÉŌļ
C£®ĖįŹ½µĪ¶Ø¹ÜŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§
D£®¶ĮČ”ŃĪĖįĢå»żŹ±£¬æŖŹ¼ŃöŹÓ¶ĮŹż£¬µĪ¶Ø½įŹųŹ±ø©ŹÓ¶ĮŹż
(3)ČōµĪ¶ØæŖŹ¼ŗĶ½įŹųŹ±£¬ĖįŹ½µĪ¶Ø¹ÜÖŠµÄŅŗĆęČēĶ¼ĖłŹ¾£¬ŌņĘšŹ¼¶ĮŹżĪŖ_______mL£¬ÖÕµć¶ĮŹżĪŖ_______mL£¬ĖłÓĆŃĪĖįČÜŅŗµÄĢå»żĪŖ_______mL”£
(4)Ä³Ń§Éśøł¾Ż3“ĪŹµŃé·Ö±š¼ĒĀ¼ÓŠ¹ŲŹż¾ŻČēĻĀ±ķ£ŗ
µĪ¶Ø “ĪŹż | “ż²āNaOHČÜŅŗµÄĢå»ż/mL | 0.100 0 mol”¤L£1 ŃĪĖįµÄĢå»ż/mL | ||
µĪ¶ØĒ°¶ĮŹż | µĪ¶Øŗó¶ĮŹż | ČÜŅŗĢå»ż/mL | ||
µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |
ŅĄ¾ŻÉĻ±ķŹż¾ŻĮŠŹ½¼ĘĖćøĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČ__________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŅ¹śæĘŃŠČĖŌ±ŅŌZnŗĶ¼ā¾§ŹÆŠĶĆĢĖįŠæ£ØZnMn2O4£©ĪŖµē¼«²ÄĮĻ£¬ŃŠÖĘ³öŅ»ÖÖĖ®ĻµŠæĄė×Óµē³Ų”£øƵē³ŲµÄ×Ü·“Ó¦·½³ĢŹ½£ŗxZn + Zn1xMn2O4![]() ZnMn2O4£Ø0 < x < 1£©”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
ZnMn2O4£Ø0 < x < 1£©”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A. ³äµēŹ±£¬Zn2+ĻņZnMn2O4µē¼«ĒØŅĘ
B. ³äµēŹ±£¬Ńō¼«·“Ó¦£ŗZnMn2O4 2xe”Ŗ£½Zn1-xMn2O4+xZn2+
C. ·ÅµēŹ±£¬Ćæ×ŖŅĘ1mol e-£¬ZnMn2O4µē¼«ÖŹĮæŌö¼Ó65g
D. ³ä·Åµē¹ż³ĢÖŠ£¬Ö»ÓŠZnŌŖĖŲµÄ»ÆŗĻ¼Ū·¢Éś±ä»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©ÅäĘ½·½³ĢŹ½ KMnO4+ HCl(ÅØ)£ KCl+ MnCl2+ Cl2”ü+ H2O£¬²¢ÓĆĖ«Ļß·Ø·ÖĪö“Ė·“Ó¦£»___”£
£Ø2£©Öø³ö___ŹĒŃõ»Æ¼Į£¬___ŌŖĖŲ±»Ńõ»Æ£¬Ńõ»Æ²śĪļŹĒ___£¬“Ė·“Ó¦ÖŠ£¬HCl±ķĻֵĊŌÖŹÓŠ___ŠŌŗĶ___ŠŌ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄūÓĆ»ÆѧŌĄķæÉŅŌ¶Ō¹¤³§ÅŷŵķĻĖ®”¢·ĻŌüµČ½ųŠŠÓŠŠ§¼ģ²āÓėŗĻĄķ“¦Ąķ”£Ä³¹¤³§¶ŌÖĘøļ¹¤ŅµĪŪÄąÖŠCr(¢ó)µÄ“¦Ąķ¹¤ŅÕĮ÷³ĢČēĶ¼£ŗ
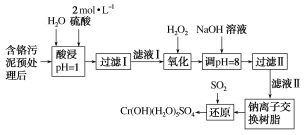
ŅŃÖŖ£ŗ¢ŁĮņĖį½žČ”ŅŗÖŠµÄ½šŹōĄė×ÓÖ÷ŅŖŹĒCr3£«£¬Ęä“ĪŹĒFe3£«”¢Al3£«”¢Ca2£«ŗĶMg2£«”£
¢Ś³£ĪĀĻĀ£¬²æ·ÖŃōĄė×ÓŅŌĒāŃõ»ÆĪļŠĪŹ½³ĮµķŹ±ČÜŅŗµÄpHČē±ķ£ŗ
¢ŪCr(OH)(H2O)5SO4ŹĒÄŃČÜĪļ”£
ŃōĄė×Ó | Fe3£« | Mg2£« | Al3£« | Cr3£« | |
³ĮµķĶźČ«Ź±µÄpH | 3.7 | 11.1 | 5.4(£¾8Čܽā) | 9(£¾9)Čܽā |
£Ø1£©ŹµŃéŹŅÓĆ18.4mol”¤L£1µÄÅØĮņĖįÅäÖĘ480 mL2mol”¤L£1µÄĮņĖį£¬ŠčŅŖĮæČ”ÅØĮņĖį___mL£»ÅäÖĘŹ±ĖłÓĆ²£Į§ŅĒĘ÷³żĮæĶ²”¢ÉÕ±ŗĶ²£Į§°ōĶā£¬»¹Šč____”£
£Ø2£©H2O2µÄ×÷ÓĆŹĒ½«ĀĖŅŗ¢ńÖŠµÄCr3£«×Ŗ»ÆĪŖCr2O72-£¬Š“³ö“Ė·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_____”£
£Ø3£©¼ÓČėNaOHČÜŅŗŹ¹ČÜŅŗ³Ź¼īŠŌ£¬¼ČæÉŅŌ³żČ„ijŠ©ŌÓÖŹĄė×Ó£¬Ķ¬Ź±ÓÖæÉŅŌ½«Cr2O72-×Ŗ»ÆĪŖ___(ĢīĪ¢Į£µÄ»ÆѧŹ½)”£
£Ø4£©ÄĘĄė×Ó½»»»Ź÷Ö¬µÄ·“Ó¦ŌĄķĪŖMn£«£«nNaR=MRn£«nNa£«£¬ŌņĄūÓĆÄĘĄė×Ó½»»»Ź÷Ö¬æɳżČ„ĀĖŅŗ¢ņÖŠµÄ½šŹōŃōĄė×ÓÓŠ___”£
£Ø5£©Š“³öÉĻŹöĮ÷³ĢÖŠÓĆSO2½ųŠŠ»¹ŌŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__”£
£Ø6£©³ĮµķµĪ¶Ø·ØŹĒ²ā¶ØĮ£×ÓÅØ¶ČµÄ·½·ØÖ®Ņ»£¬ĪŖĮĖ²ā¶Øij·ĻĖ®ÖŠSCN£µÄÅØ¶Č£¬æÉÓƱź×¼AgNO3ČÜŅŗµĪ¶Ø“ż²āŅŗ£¬ŅŃÖŖ£ŗ
ŅųŃĪŠŌÖŹ | AgCl | AgI | AgCN | Ag2CrO4 | AgSCN |
ŃÕÉ« | °× | »Ę | °× | שŗģ | °× |
Ksp | 1.8”Į10£10 | 8.3”Į10£17 | 1.2”Į10£16 | 3.5”Į10£11 | 1.0”Į10£12 |
µĪ¶ØŹ±æÉŃ”ĪŖµĪ¶ØÖøŹ¾¼ĮµÄŹĒ___(Ģī±ąŗÅ)£¬µĪ¶ØÖÕµćµÄĻÖĻóŹĒ___”£
A£®NaCl B£®K2CrO4 C£®KI D£®NaCN
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹĒ»Æѧъ¾æµÄ»ł“””£ĻĀĮŠ¹ŲÓŚø÷ŹµŃé×°ÖƵĊšŹöÕżČ·µÄŹĒ£Ø £©

A. ×°ÖĆ¢Ł³£ÓĆÓŚ·ÖĄė»„²»ĻąČܵÄŅŗĢå»ģŗĻĪļ
B. ×°ÖĆ¢ŚæÉÓĆÓŚĪüŹÕNH3»ņHClĘųĢ壬²¢·ĄÖ¹µ¹Īü
C. ×°ÖĆ¢ÜæÉÓĆÓŚøÉŌļ”¢ŹÕ¼ÆĀČ»ÆĒā£¬²¢ĪüŹÕ¶ąÓąµÄĀČ»ÆĒā
D. ×°ÖĆ¢ŪæÉÓĆÓŚŹÕ¼ÆH2”¢CO2”¢Cl2”¢NH3µČĘųĢå
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖÓŠĻĀĮŠŹ®ÖÖĪļÖŹ£ŗ¢ŁŅŗĢ¬HCl ¢ŚNaHCO3 ¢ŪNaClČÜŅŗ ¢ÜCO2 ¢ŻÕįĢĒ¾§Ģå ¢ŽBa(OH)2 ¢ßŗģŗÖÉ«µÄĒāŃõ»ÆĢś½ŗĢå ¢ąNH3H2O ¢įæÕĘų ¢āAl2(SO4)3
£Ø1£©ÉĻŹöŹ®ÖÖĪļÖŹÖŠÓŠĮ½ÖÖĪļÖŹŌŚĖ®ČÜŅŗÖŠæÉ·¢Éś·“Ó¦£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗH++OH£=H2O£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________________________”£
£Ø2£©¢ŚŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ______________________________”£
£Ø3£©ĪøŅŗÖŠŗ¬ÓŠŃĪĖį£¬ĪøĖį¹ż¶ąµÄČĖ³£ÓŠĪøĢŪÉՊĵÄøŠ¾õ£¬Ņ×ĶĀĖįĖ®£¬·žÓĆŹŹĮæµÄŠ”ĖÕ“ņ£ØNaHCO3£©£¬ÄÜÖĪĮĘĪøĖį¹ż¶ą£¬ĒėŠ“³öĘä·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________£»Čē¹ū²”ČĖĶ¬Ź±»¼ĪøĄ£Ńń£¬ĪŖ·ĄĪø±Ś“©æ×£¬²»ÄÜ·žÓĆŠ”ĖÕ“ņ£¬“ĖŹ±×īŗĆÓĆŗ¬Al(OH)3µÄĪøŅ©£ØČēĪøŹęĘ½£©£¬ĖüÓėĪøĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½________”£
£Ø4£©Š“³öĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ĻņBa(OH)2ČÜŅŗÖŠÖšµĪ¼ÓČėĮņĖįČÜŅŗ___________________________”£
ĻņBa(OH)2ČÜŅŗÖŠĶØČė¹żĮæµÄCO2_______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓÉA”¢B”¢C”¢DĖÄÖÖ½šŹō°“ĻĀ±ķ֊װÖĆ½ųŠŠŹµŃ飬øł¾ŻŹµŃéĻÖĻóĢī±ķ£ŗ
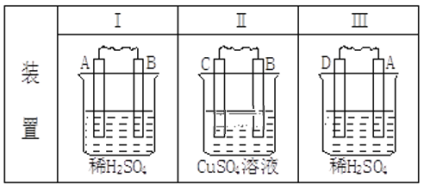
ĻÖĻó | £ØI£©½šŹōAÖš½„Čܽā | £Ø¢ņ£©CµÄÖŹĮæŌö¼Ó | £Ø¢ó£©AÉĻÓŠĘųĢå²śÉś |
ĢīÕż¼«·“Ó¦Ź½ | ___ | ___ | |
ĖÄÖÖ½šŹō»ī¶ÆŠŌÓÉĒæµ½ČõµÄĖ³Šņ£ŗ___”£ | |||
Čō×°ÖĆ£Ø¢ó£©ÖŠA¼«²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ224mL£¬ŌņĶعżµ¼ĻßÖŠµÄµē×ÓµÄĪļÖŹµÄĮæĪŖ___mol”£ | |||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČ”28gĢś½«ĘäĶ¶Čė×ćĮæĻ”ĮņĖįÖŠĶźČ«Čܽā£¬
£Ø1£©²śÉśĒāĘų¶ąÉŁĢå»ż£æ
£Ø2£©£Ø±ź×¼×“æö£©²Ī¼Ó·“Ó¦µÄĮņĖįĪļÖŹµÄĮæŹĒ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com