
(1)ʵ��ʱ����ͬѧѡ����������Һ���Լ�A����ͬѧѡ���Ȼ�����Һ���Լ�A������_______ͬѧ��ѡ���Լ�A��������������___________________________________��
(2)���¶������Լ�Aѡ����ȷʱ���е�ʵ�飺
��Ϊ���г������ܽ⡢��Ӧ�����ˡ�ϴ�ӳ����ĸ����������������������������ƽ�����룬�ձ���������������̨����������ͷ�ιܡ���Ͳ�����л�ȱ��һ���ر���������___________��
�����������ó���δ��ϴ�Ӻ�ɳ�������ý����__________(�ƫ�ߡ���ƫ�͡�)��
����ʵ���û���������ΪW g�������������Ϊm g��ʵ��ⶨ���Ȼ��������������ʽ=__________��
������(1)��ͬѧѡ���Լ�A�Ǵ��ģ���ΪAgNO3�ȿ�����NH4Cl��Ӧ����AgCl��ɫ�������ֿ���(NH4)2SO4��Ӧ��������ˮ��Ag2SO4,�ﲻ��ʵ��Ŀ�ġ�
(2)�ٲ��������ܽ⡢ϴ�ӡ����˵ȸ�������ʹ�ò�������
��δ��ϴ�ӡ���ɾͳ�����ʹ�ó�����ֵ��ʵ����ֵ����������NH4Cl��������ƫ�͡�
��(NH4)2SO4+BaCl2===BaSO4��+2NH4Cl
132 233
x m g
x= ![]() g
g
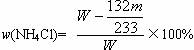
�𰸣�(1)�� ��ΪAgNO3�ȿ�����NH4Cl��Ӧ����AgCl��ɫ�������ֿ���(NH4)2SO4��Ӧ��������ˮ��Ag2SO4,�ﲻ��ʵ��Ŀ��
(2)�ٲ����� ��ƫ��
��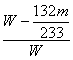 ��100%
��100%



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�����л�ѧϰ�� ���ͣ�058
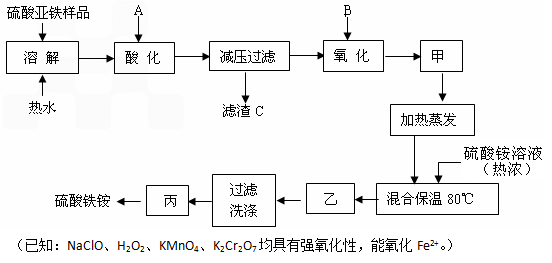
Ϊ�˲ⶨ����狀��Ȼ�炙�������Ȼ�淋������������ס�����ͬѧ��������ʾ��ʵ�鲽�����ʵ�飺
������Ʒ���ܽ���ӹ������Լ�A�����˳�����B��ϴ�ӳ�������ɳ���������ʵ�����ݵó������
(1)ʵ��ʱ����ͬѧѡ��![]() ��Һ���Լ�A����ͬѧѡ���Ȼ�����Һ���Լ�A��������λͬѧ��ѡ����Լ�A����������ԭ����________��
��Һ���Լ�A����ͬѧѡ���Ȼ�����Һ���Լ�A��������λͬѧ��ѡ����Լ�A����������ԭ����________��
(2)���������ó���δ��ϴ�Ӽ���ɳ������ⶨ�����(ƫ�ߡ�ƫ�͡�����)
________��
(3)���������Լ�A�Ƿ�����ķ�����________��
(4)��ʵ���в����Ʒ����Ϊw g������������Ϊm g���г�ʵ��ⶨ����ļ���ʽ���Ȼ�淋���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˲ⶨ����狀��Ȼ�炙�������Ȼ�淋������������ס�����λͬѧ������ʵ�鲽�����ʵ�飺������Ʒ���ܽ���ӹ����Լ�A�����˳��ֳ���B��ϴ�ӳ�������ɳ���������ʵ�����ݲ��ó������
(1)ʵ��ʱ����ͬѧѡ����������Һ���Լ�A����ͬѧѡ���Ȼ�����Һ���Լ�A������ͬѧ��ѡ���Լ�A��������������________________��
��2�����¶������Լ�Aѡ����ȷʱ���е�ʵ�飺
��Ϊ���г������ܽ⡢��Ӧ�����ˡ�ϴ�ӳ����ĸ����������������������������ƽ�����룬�ձ���������������̨����������ͷ�ιܡ���Ͳ�����л�ȱ��һ���ر���������_____________��
�����������ó���δ��ϴ�Ӻ�ɳ�������ý����________���ƫ�ߡ���ƫ�͡�����
����ʵ���û���������ΪW g�������������Ϊm g��ʵ��ⶨ���Ȼ��������������ʽ=_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com