
 ЁЃ
ЁЃ ЁЃ
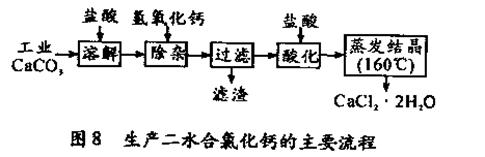
ЁЃ ВтЖЈНсЙћЦЋДѓЃЈ2ЗжЃЌКЯРэдђИјЗжЃЉ
ВтЖЈНсЙћЦЋДѓЃЈ2ЗжЃЌКЯРэдђИјЗжЃЉ

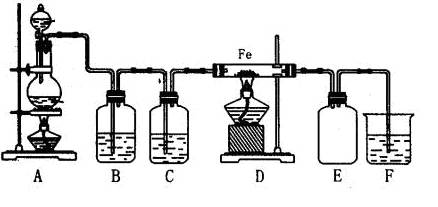
 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт


ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт

| бЁЯю | вЉЦЗКЭЪдМС | ЯДЦјЦПжаЪдМС | МЏЦјЦПжаЦјЬх |
| A | ТШЫсМиКЭMnO2 | ХЈСђЫс | O2 |
| B | ЪЏЛвЪЏКЭЯЁбЮЫс | БЅКЭNaHCO3ШмвК | CO2 |
| C | ZnКЭЯЁСђЫс | ХЈСђЫс | H2 |
| D | Na2CO3КЭХЈбЮЫс | ХЈЯѕЫс | CO2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎввЫсввѕЅ(ввЫс)ЃЌМгNaOHШмвКЁЂЗжвК |
| BЃЎИЃЖћТэСж(ввЫс)ЃЌМгNa2CO3ШмвКЁЂЗжвК |
| CЃЎфхввЭщЃЈввДМЃЉЃЌМгЫЎеёЕДЁЂЗжвК |
| DЃЎБН(БНЗг)ЃЌМгфхЫЎеёЕДЁЂЙ§ТЫ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎжЦCl2ЃКMnO2ЁЂбЮЫсЃЈХЈЃЉ | BЃЎжЦH2ЃКСђЫсЃЈЯЁЃЉЁЂZn |
| CЃЎжЦO2ЃК MnO2ЁЂH2O2 | DЃЎжЦCO2ЃКСђЫсЃЈЯЁЃЉЁЂCaCO3 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт


ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
 1)ЯТЭМЪЧгУKMnO4гыХЈбЮЫсЗДгІжЦШЁЪЪСПТШЦјЕФМђвззАжУЁЃ
1)ЯТЭМЪЧгУKMnO4гыХЈбЮЫсЗДгІжЦШЁЪЪСПТШЦјЕФМђвззАжУЁЃ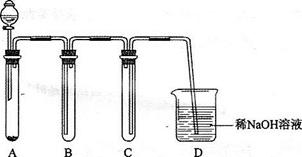

ВщПДД№АИКЭНтЮі>>
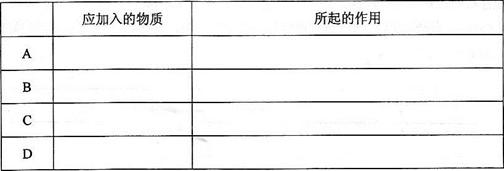
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
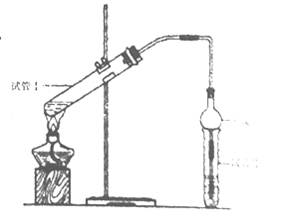
| ЪЕбщБрКХ | ЪдЙмЂёжаЕФЪдМС | ЪдЙмЂђжаЕФЪдМС | ВтЕУгаЛњВуЕФКёЖШ/cm |
| A | 2mLввДМЁЂ2 mLввЫсЁЂ1 mL 18mol/LХЈСђЫс | БЅКЭЬМЫсФЦШмвК | 5.0 |
| B | 3 mLввДМЁЂ2 mLввЫс | 0.1 | |
| C | 3 mLввДМЁЂ2 mLввЫсЁЂ6 mL 3mol/LСђЫс | 1.2 | |
| D | 3 mLввДМЁЂ2 mLввЫсЁЂбЮЫс | 1.2 |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com