
£Ø16·Ö£©
ŅŃÖŖCH3COOH£Ø·Ö×ÓĮæĪŖ60£©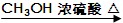 CH3COOCH3£Ø·Ö×ÓĮæĪŖ74£©
CH3COOCH3£Ø·Ö×ÓĮæĪŖ74£©
ĻÖÓŠÖ»ŗ¬C”¢H”¢OµÄ»ÆŗĻĪļA”ŖF(ĻąĶ¬µÄ¹ŁÄÜĶÅĮ¬½ÓŌŚ²»Ķ¬Ģ¼Ō×ÓÉĻ£¬AµÄŅ»Ā±“śĪļ
Ö»ÓŠŅ»ÖÖ)£¬ÓŠ¹ŲĖüĆĒµÄijŠ©ŠÅĻ¢£¬ŅŃ×¢Ć÷ŌŚĻĀĆęµÄ·½æņÄŚ£ŗ
£Ø1£©ŌŚ»ÆŗĻĪļA”«FÖŠ£¬¾ßõ„µÄ½į¹¹µÄ»ÆŗĻĪļŹĒ£ØĢīŠ“¹ŁÄø“śŗÅ£©£ŗ ”£
£Ø2£©AŗĶCµÄ½į¹¹¼ņŹ½£ŗ
A ӣC ӣ
£Ø3£©Š“³öAÓėŠĀÖʵÄĒāŃõ»ÆĶ×ĒŅŗ¹²ČȵĻÆѧ·½³ĢŹ½£ŗ ”£
£Ø4£©Š“³öFÓė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ¹²ČȵĻÆѧ·½³ĢŹ½£ŗ ”£
£Ø5£©GŹĒBµÄĶ¬·ÖŅģ¹¹Ģ壬ĒŅG±ŲŠėĀś×ćČēĻĀĢõ¼ž£ŗ
¢Ł GŹĒ·¼Ļć×å»ÆŗĻĪļÖ»ÓŠŅ»ÖÖ¹ŁÄÜĶÅ”£
¢Ś1molGĒ”ŗĆÄÜÓė3molĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦
¢ŪG·Ö×ÓÖŠ²»ŗ¬¼×»ł£¬ĒŅĶ¬Ņ»ÖÖ¹ŁÄÜĶŲ»Į¬½ÓŌŚŅ»øöĢ¼Ō×ÓÉĻ”£
ŌņĀś×ćĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåÓŠ£ŗ ÖÖ”£ĒėČĪŠ“Į½ÖÖ£ŗ
ӣ
£Ø1£©BCEF
(2) 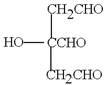 Ӣ
Ӣ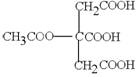
£Ø3£© +6Cu(OH)2
+6Cu(OH)2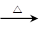
 +3Cu2O”ż+6H2O.
+3Cu2O”ż+6H2O.
£Ø4£©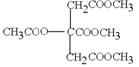 £«4NaOH
£«4NaOH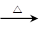 CH3COONa£«3CH3OH£«
CH3COONa£«3CH3OH£«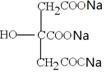
£Ø5£©6
 Ӣ
”¢ µČ
µČ
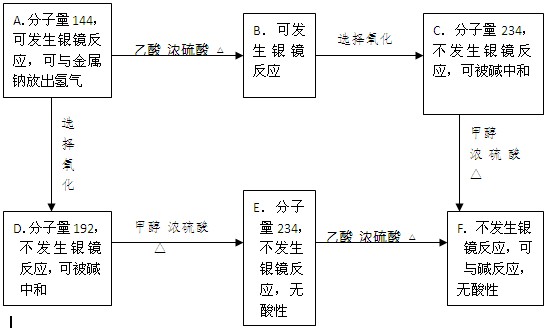
”¾½āĪö”æAŗĶŅŅĖįõ„»ÆÉś³ÉB£¬AÖŠŗ¬ÓŠōĒ»ł£¬BµÄ·Ö×ÓĮæŹĒ186£¬BÖŠµÄČ©»ł±»Ńõ»ÆÉś³ÉōČ»ł£¬ŅņĪŖ1øöČ©»łÉś³ÉōČ»ł£¬·Ö×ÓĮæŌö¼Ó16£¬C±ČB¶ą³ö48£¬ĖłŅŌBÖŠŗ¬ÓŠ3øöČ©»ł£¬ŌņAÖŠŅ²ŗ¬ÓŠ3øöČ©»ł”£AÖŠĻąĶ¬µÄ¹ŁÄÜĶÅĮ¬½ÓŌŚ²»Ķ¬Ģ¼Ō×ÓÉĻ£¬ĒŅAµÄŅ»Ā±“śĪļÖ»ÓŠŅ»ÖÖ
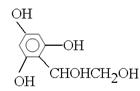
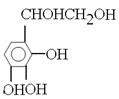
ĖłŅŌøł¾ŻAµÄ·Ö×ÓĮææÉµĆ³öĘä½į¹¹¼ņŹ½ĪŖ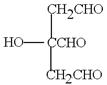 £¬ŌņB”¢C”¢D”¢E”¢F·Ö±šĪŖ
£¬ŌņB”¢C”¢D”¢E”¢F·Ö±šĪŖ ”¢
Ӣ 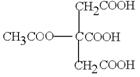 Ӣ
Ӣ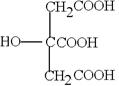 Ӣ
Ӣ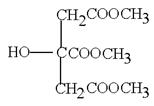 Ӣ
”¢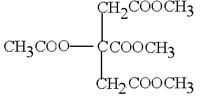 ”£1molGĒ”ŗĆÄÜÓė3molĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬ĖµĆ÷ŗ¬ÓŠōČ»ł»ņ·ÓōĒ»ł£¬GŹĒ·¼Ļć×å»ÆŗĻĪļÖ»ÓŠŅ»ÖÖ¹ŁÄÜĶÅ£¬Ņņ“Ė¹ŁÄÜĶÅŹĒōĒ»ł£¬ÓÖŅņĪŖG·Ö×ÓÖŠ²»ŗ¬¼×»ł£¬ĒŅĶ¬Ņ»ÖÖ¹ŁÄÜĶŲ»Į¬½ÓŌŚŅ»øöĢ¼Ō×ÓÉĻ£¬ĖłŅŌ±½»·ÉĻĮ¬½ÓµÄČ”“ś»łŹĒ3øö·ÓōĒ»ł£¬1øö£CHOHCH2OH”£Ņņ“Ė¹²ÓĆ6ÖÖ”£ĄżČē
”£1molGĒ”ŗĆÄÜÓė3molĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬ĖµĆ÷ŗ¬ÓŠōČ»ł»ņ·ÓōĒ»ł£¬GŹĒ·¼Ļć×å»ÆŗĻĪļÖ»ÓŠŅ»ÖÖ¹ŁÄÜĶÅ£¬Ņņ“Ė¹ŁÄÜĶÅŹĒōĒ»ł£¬ÓÖŅņĪŖG·Ö×ÓÖŠ²»ŗ¬¼×»ł£¬ĒŅĶ¬Ņ»ÖÖ¹ŁÄÜĶŲ»Į¬½ÓŌŚŅ»øöĢ¼Ō×ÓÉĻ£¬ĖłŅŌ±½»·ÉĻĮ¬½ÓµÄČ”“ś»łŹĒ3øö·ÓōĒ»ł£¬1øö£CHOHCH2OH”£Ņņ“Ė¹²ÓĆ6ÖÖ”£ĄżČē ”¢
”¢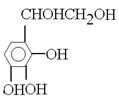 µČ”£
µČ”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¢ń”¢£Ø1£©ŌŚ25”ęĢõ¼žĻĀ½«pH=3µÄ“×ĖįĻ”ŹĶ100±¶ŗóČÜŅŗµÄpHĪŖ
¢ń”¢£Ø1£©ŌŚ25”ęĢõ¼žĻĀ½«pH=3µÄ“×ĖįĻ”ŹĶ100±¶ŗóČÜŅŗµÄpHĪŖ²éæ““š°øŗĶ½āĪö>>
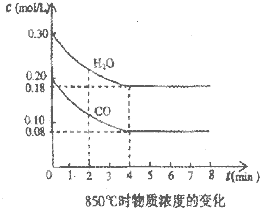
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗɽĪ÷Ź”Ģ«ŌĪåÖŠ2011£2012ѧğø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧĄķæĘŹŌĢā(ČĖ½Ģ°ę) ĢāŠĶ£ŗ022
ĻÖÓŠĻĀĮŠĪļÖŹ£ŗ
¢ŁH2S”¢
¢ŚNH3”¢
¢ŪH2SO4”¢
¢ÜNaHCO3”¢
¢ŻCH3COOH”¢
¢ŽKNO3ČÜŅŗ”¢
¢ßŃĪĖį”¢
¢ą¾Ę¾«”¢
¢į¶žŌŖĖįH2AµČ£®
Ēė»Ų“šĻĀĮŠĪŹĢā(ĒėĢīČėŠņŗÅ)£®
(1)ĘäÖŠŅ»¶ØŹōÓŚČõµē½āÖŹµÄŹĒ________£®²»ÄÜČ·¶ØµÄŹĒ________£¬ĒėÉč¼ĘŅ»øöŹµŃéŅŌÖ¤Ć÷ĖüŹĒĒæµē½āÖŹ»¹ŹĒČõµē½āÖŹ£®ÄćµÄŹµŃéŗĶ½įĀŪŹĒ________£»
(2)H2SŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ________£»NaHCO3ŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ________£»ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A![]() H+£«HA££»HA£
H+£«HA££»HA£![]() H+£«A2££®ŹŌÅŠ¶ĻH2AŹĒ________µē½āÖŹ(Ģī”°Ēæ”±»ņ”°Čõ”±)£®
H+£«A2££®ŹŌÅŠ¶ĻH2AŹĒ________µē½āÖŹ(Ģī”°Ēæ”±»ņ”°Čõ”±)£®
(3)2 mol/LµÄŃĪĖįŗĶ2 mol/LµÄ“×Ėįø÷100 ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»ż£¬V(ŃĪĖį)________V(“×Ėį)(Ģī£¾”¢£½»ņ£¼)£»ĒāĄė×ÓÅضČĻąµČµÄŃĪĖįŗĶ“×Ėįø÷100 ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»żV(ŃĪĖį)________V(“×Ėį)(Ģī£¾”¢£½»ņ£¼)£»ĄķÓÉŹĒ________£»
(4)ŅŃÖŖCH3COO££«H+![]() CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ________
CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ________
A£®¼ÓNaOH
B£®¼ÓŃĪĖį
C£®¼ÓĖ®
D£®ÉżøßĪĀ¶Č
ŅŃÖŖCH3COOHŌŚČܼĮAÖŠæÉŅŌČ«²æµēĄė£¬ŃĪ²»ČܽāÓŚAČܼĮ£®ŌņCH3COOHŌŚČܼĮAÖŠµÄµēĄė·½³ĢŹ½ĪŖ________£»CH3COOHŗĶNa2CO3ŌŚČܼĮAÖŠÉś³ÉCO2·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ÄźÉ½Ī÷Ź”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»Æѧ£ØĄķ£©ŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø18·Ö£©”¢ĻÖÓŠĻĀĮŠĪļÖŹ£ŗ¢ŁH2S”¢¢ŚNH3”¢¢ŪH2SO4”¢¢ÜNaHCO3”¢
¢ŻCH3COOH”¢¢ŽKNO3ČÜŅŗ”¢¢ßŃĪĖį”¢¢ą¾Ę¾«”¢¢į¶žŌŖĖįH2AµČ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ØĒėĢīČėŠņŗÅ£©”£
(1)ĘäÖŠŅ»¶ØŹōÓŚČõµē½āÖŹµÄŹĒ ”£²»ÄÜČ·¶ØµÄŹĒ £¬ĒėÉč¼ĘŅ»øöŹµŃéŅŌÖ¤Ć÷ĖüŹĒĒæµē½āÖŹ»¹ŹĒČõµē½āÖŹ”£ÄćµÄŹµŃéŗĶ½įĀŪŹĒ
£»
£Ø2£©H2SŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ __________£»
NaHCO3ŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ £»
 ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A == H++HA-£» HA-
H++A2-”£ŹŌÅŠ¶ĻH2AŹĒ µē½āÖŹ£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A == H++HA-£» HA-
H++A2-”£ŹŌÅŠ¶ĻH2AŹĒ µē½āÖŹ£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
£Ø3£©2mol/LµÄŃĪĖįŗĶ2mol/LµÄ“×Ėįø÷100ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»ż£¬V(ŃĪĖį) V(“×Ėį)£ØĢī>”¢=»ņ<£©£»ĒāĄė×ÓÅضČĻąµČµÄŃĪĖįŗĶ“×Ėįø÷100ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»żV(ŃĪĖį) V(“×Ėį) £ØĢī>”¢=»ņ<£©£»ĄķÓÉŹĒ
£»
 £Ø4£©ŅŃÖŖCH3COO-+H+
CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ£Ø £©
£Ø4£©ŅŃÖŖCH3COO-+H+
CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ£Ø £©
A.¼ÓNaOH B.¼ÓŃĪĖį C.¼ÓĖ® D.ÉżøßĪĀ¶Č
ŅŃÖŖCH3COOHŌŚČܼĮAÖŠæÉŅŌČ«²æµēĄė£¬ŃĪ²»ČܽāÓŚAČܼĮ”£ŌņCH3COOHŌŚČܼĮAÖŠµÄµēĄė·½³ĢŹ½ĪŖ £»CH3COOHŗĶNa2CO3ŌŚČܼĮAÖŠÉś³ÉCO2·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø18·Ö£©”¢ĻÖÓŠĻĀĮŠĪļÖŹ£ŗ¢ŁH2S”¢¢ŚNH3”¢¢ŪH2SO4”¢¢ÜNaHCO3”¢
¢ŻCH3COOH”¢¢ŽKNO3ČÜŅŗ”¢¢ßŃĪĖį”¢¢ą¾Ę¾«”¢¢į¶žŌŖĖįH2AµČ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ØĒėĢīČėŠņŗÅ£©”£
(1)ĘäÖŠŅ»¶ØŹōÓŚČõµē½āÖŹµÄŹĒ ”£²»ÄÜČ·¶ØµÄŹĒ £¬ĒėÉč¼ĘŅ»øöŹµŃéŅŌÖ¤Ć÷ĖüŹĒĒæµē½āÖŹ»¹ŹĒČõµē½āÖŹ”£ÄćµÄŹµŃéŗĶ½įĀŪŹĒ
£»
£Ø2£©H2SŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ __________£»
NaHCO3ŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ £»
 ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A == H++HA-£» HA- H++A2-”£ŹŌÅŠ¶ĻH2AŹĒ µē½āÖŹ£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A == H++HA-£» HA- H++A2-”£ŹŌÅŠ¶ĻH2AŹĒ µē½āÖŹ£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
£Ø3£©2mol/LµÄŃĪĖįŗĶ2mol/LµÄ“×Ėįø÷100ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»ż£¬V(ŃĪĖį) V(“×Ėį)£ØĢī>”¢=»ņ<£©£»ĒāĄė×ÓÅضČĻąµČµÄŃĪĖįŗĶ“×Ėįø÷100ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»żV(ŃĪĖį) V(“×Ėį) £ØĢī>”¢=»ņ<£©£»ĄķÓÉŹĒ
£»
![]() £Ø4£©ŅŃÖŖCH3COO-+H+ CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ£Ø £©
£Ø4£©ŅŃÖŖCH3COO-+H+ CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ£Ø £©
A.¼ÓNaOH B.¼ÓŃĪĖį C.¼ÓĖ® D.ÉżøßĪĀ¶Č
ŅŃÖŖCH3COOHŌŚČܼĮAÖŠæÉŅŌČ«²æµēĄė£¬ŃĪ²»ČܽāÓŚAČܼĮ”£ŌņCH3COOHŌŚČܼĮAÖŠµÄµēĄė·½³ĢŹ½ĪŖ £»CH3COOHŗĶNa2CO3ŌŚČܼĮAÖŠÉś³ÉCO2·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ÄźÉ½Ī÷Ź”Ģ«ŌĪåÖŠø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»Æѧ£ØĄķ£©ŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø18·Ö£©”¢ĻÖÓŠĻĀĮŠĪļÖŹ£ŗ¢ŁH2S”¢¢ŚNH3”¢¢ŪH2SO4”¢¢ÜNaHCO3”¢
¢ŻCH3COOH”¢¢ŽKNO3ČÜŅŗ”¢¢ßŃĪĖį”¢¢ą¾Ę¾«”¢¢į¶žŌŖĖįH2AµČ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ØĒėĢīČėŠņŗÅ£©”£
(1)ĘäÖŠŅ»¶ØŹōÓŚČõµē½āÖŹµÄŹĒ ”£²»ÄÜČ·¶ØµÄŹĒ £¬ĒėÉč¼ĘŅ»øöŹµŃéŅŌÖ¤Ć÷ĖüŹĒĒæµē½āÖŹ»¹ŹĒČõµē½āÖŹ”£ÄćµÄŹµŃéŗĶ½įĀŪŹĒ
£»
£Ø2£©H2SŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ __________£»
NaHCO3ŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖ £» ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A ="=" H++HA-£» HA- H++A2-”£ŹŌÅŠ¶ĻH2AŹĒ µē½āÖŹ£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
ŅŃÖŖH2AµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A ="=" H++HA-£» HA- H++A2-”£ŹŌÅŠ¶ĻH2AŹĒ µē½āÖŹ£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
£Ø3£©2mol/LµÄŃĪĖįŗĶ2mol/LµÄ“×Ėįø÷100ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»ż£¬V(ŃĪĖį) V(“×Ėį)£ØĢī>”¢=»ņ<£©£»ĒāĄė×ÓÅضČĻąµČµÄŃĪĖįŗĶ“×Ėįø÷100ml£¬·Ö±šÓė¹żĮæµÄZn·“Ó¦£¬Éś³ÉH2µÄĢå»żV(ŃĪĖį) V(“×Ėį) £ØĢī>”¢=»ņ<£©£»ĄķÓÉŹĒ
£» £Ø4£©ŅŃÖŖCH3COO-+H+ CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ£Ø £©
£Ø4£©ŅŃÖŖCH3COO-+H+ CH3COOH£»ĻÖŅŖŹ¹Ę½ŗāĻņÓŅŅʶÆĒŅĒāĄė×ÓÅضČŌö“ó£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ£Ø £©
| A£®¼ÓNaOH | B£®¼ÓŃĪĖį | C£®¼ÓĖ® | D£®ÉżøßĪĀ¶Č |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com