
ij��Ͻ�����ĩ����Mg�⣬������Al��Zn�е�һ�ֻ����֣���������10%���ϡ�ij�о�С�����ʵ��̽���û�Ͻ�����ĩ������пԪ�صĴ��ڡ������Լ�����Ʒ��pH��ֽ��ϡH2SO4��NaOH��Һ��ϡNH3��H2O����С��̽���������£�
�� �������ϣ�
��þ������п��������ɫ�Ľ���
��п(Zn)������NaOH��Һ��Ӧ����H2
��Zn(OH)2Ϊ��ɫ���壬������ˮ��������ǿ�NH3��H2O
��Zn2+���γ��������Zn(NH3)42+�����������ǿ��ֽ�����Zn2+��NH4+
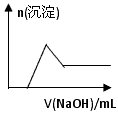
�� ������裺
��1������٣��û�Ͻ�����ĩ�г�þ�����________Ԫ��
����ڣ��û�Ͻ�����ĩ�г�þ�����________Ԫ��
����ۣ��û�Ͻ�����ĩ�г�þ���������пԪ��
�� ʵ��̽������ͬѧ���ڼ�������ʵ�鷽�����£�

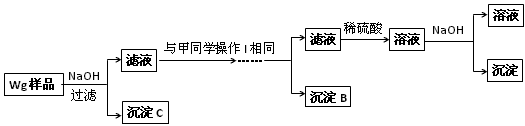
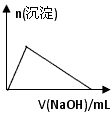
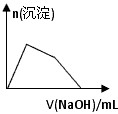
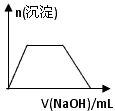
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��þ������п��������ɫ�Ľ��� ��п��Zn��������NaOH��Һ��Ӧ����H2 ��Zn��OH��2Ϊ��ɫ���壬������ˮ��������ǿ�NH3?H2O ��Zn2+���γ��������[Zn��NH3��4]2+�����������ǿ��ֽ�����Zn2+��NH4+��������裺 ��1������٣��û�Ͻ�����ĩ�г�þ����� Al Al Ԫ������ڣ��û�Ͻ�����ĩ�г�þ����� Zn Zn Ԫ������ۣ��û�Ͻ�����ĩ�г�þ���������пԪ�� ��ʵ��̽���� ��ͬѧ���ڼ�������ʵ�鷽�����£���ͬѧͬ�����ڼ���������һʵ�鷽�����£� 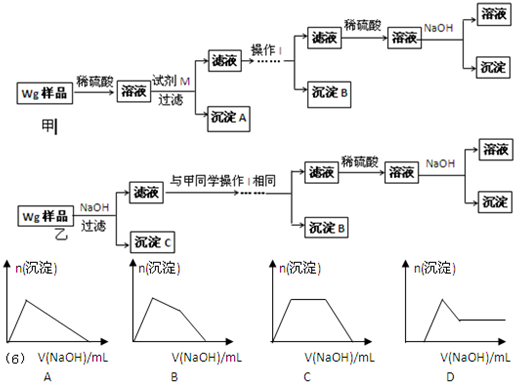 ��2���Լ�M�� NaOH��Һ NaOH��Һ ������B��Al��OH��3 Al��OH��3 ����3����ͬѧ��Ϊ��ͬѧ�ķ����ȼ�ͬѧ�ĺã������� �������Լ�Լ� �������Լ�Լ� ����4����ͬѧ�о��˼ס�����ͬѧ�ķ�����������һ�ַ����Ļ����������㷽���ⶨ��Wg��Ʒ�н���þ��Mg�������������ķ����� ������Cϴ�ӡ�С�ĸ������� ������Cϴ�ӡ�С�ĸ������� ����5�����������Ҫ�����ǣ�����Һ����μ��� ϡ���� ϡ���� ��ֱ�����ɵij����պ��ܽ⣬�ټ���������ϡ��ˮ ϡ��ˮ ����6��ij��Һ�к���Zn2+��Al3+��NH4+��SO42-�����ӣ���������μ���NaOH��Һ�������ɳ��������ʵ��������NaOH��Һ�����ϵ��ͼ����ȷ���� B B ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ij��Ͻ�����ĩ����Mg�⣬������Al��Zn�е�һ�ֻ����֣��������� 10%���ϡ�ij�о�С�����ʵ��̽���û�Ͻ�����ĩ������пԪ�صĴ��ڡ� �����Լ�����Ʒ��pH��ֽ��ϡH2SO4��NaOH��Һ��ϡNH3��H2O�� ��С��̽���������£� �� �������ϣ�
�� ������裺 ��1������٣��û�Ͻ�����ĩ�г�þ�����________Ԫ�� ����ڣ��û�Ͻ�����ĩ�г�þ�����________Ԫ�� ����ۣ��û�Ͻ�����ĩ�г�þ���������пԪ�� �� ʵ��̽���� ��ͬѧ���ڼ�������ʵ�鷽�����£� ��ͬѧͬ�����ڼ���3�����һʵ�鷽�����£� ��2���Լ�M��________________������B�� �� ��3����ͬѧ��Ϊ��ͬѧ�ķ����ȼ�ͬѧ�ĺã������� �� ��4����ͬѧ�о��˼ס�����ͬѧ�ķ�����������һ�ַ����Ļ����������㷽���ⶨ��Wg��Ʒ�н���þ(Mg)�����������ķ����� �� ��5�����������Ҫ�����ǣ�����Һ����μ��� ��ֱ�����ɵij����պ��ܽ⣬�ټ��������� �� ��6��ij��Һ�к���Zn2+��Al3+��NH4+��SO42�������ӣ���������μ���NaOH��Һ�� �����ɳ��������ʵ��������NaOH��Һ�����ϵ��ͼ����ȷ����__________��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012���Ϻ���������������ѧ����ĩ������⻯ѧ�Ծ� ���ͣ������ ij��Ͻ�����ĩ����Mg�⣬������Al��Zn�е�һ�ֻ����֣���������10%���ϡ�ij�о�С�����ʵ��̽���û�Ͻ�����ĩ������пԪ�صĴ��ڡ������Լ�����Ʒ��pH��ֽ��ϡH2SO4��NaOH��Һ��ϡNH3��H2O��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�������ʡ������ѧ�ڵ��������տ��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ���� ij��Ͻ�����ĩ����Fe�⣬������Al��Zn�е�һ�ֻ����֣���������10%���ϡ�ij�о�С�����ʵ��̽���û�Ͻ�����ĩ������пԪ�صĴ��ڣ�̽���������£� ���������ϡ���Zn������NaOH��Һ��Ӧ����H2 ��Zn(OH)2Ϊ��ɫ���壬������ˮ��������ǿ�NH3��H2O ��Zn2+���γ��������[Zn(NH3)4]2+�����������ǿ������Zn2+��NH4+ ��������衿����٣��û�Ͻ�����ĩ�г�Fe�����AlԪ�� ����ڣ��û�Ͻ�����ĩ�г�Fe�����ZnԪ�� ����ۣ��û�Ͻ�����ĩ�г�Fe�����Al��ZnԪ�� ��ʵ��̽���������Լ�����Ʒ��pH��ֽ��ϡH2SO4��NaOH��Һ��ϡNH3��H2O�� ��ͬѧ���ڼ�������ʵ�鷽�����£�
��ͬѧͬ�����ڼ���������һʵ�鷽�����£�
��1��FeԪ�������ڱ��е�λ���ǵ� ���� �塣 ��2���Լ�M�� ������B�� �� ��3������Ϊ�ס���ͬѧ�ķ��� ����ס����ҡ����ȽϺã������� �� ��4�����������Ҫ�����ǣ�����Һ����μ��� ��ֱ�����ɵij����պ��ܽ⣬�ټ��������� �� ��5����ͬѧ������Cϴ�ӡ���ɡ���ȴ����أ�����Ϊm1g�������㼴�ɵõ���Ʒ��������������������Ϊ ����ǡ���ȷ��ԭ���� �� ��6����ⷨ�ƽ������Ļ�ѧ����ʽ�� �� ��Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ�ط�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�갲ʡ�����и�����ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ������ ij��Ͻ�����ĩ����Mg�����Al��Zn�е�һ�ֻ����֣���������10%���ϡ�ij�о�С�����ʵ��̽���û�Ͻ�����ĩ������пԪ�صĴ��ڡ� �����Լ�����Ʒ��pH��ֽ��ϡH2SO4��NaOH��Һ��ϡNH3��H2O�� ��С��̽���������£� ��������ϣ�
��������裺 ��1������٣��û�Ͻ�����ĩ�г�þ�������Ԫ�أ� ����ڣ��û�Ͻ�����ĩ�г�þ�����пԪ�أ� ����ۣ� �� ��ʵ��̽���� ��ͬѧ���ڼ�������ʵ�鷽�����£�
��ͬѧͬ�����ڼ���3�����һʵ�鷽�����£�
��2���Լ�M��________________������B�� �� ��3����ͬѧ��Ϊ��ͬѧ�ķ����ȼ�ͬѧ�ĺã������� �� ��4����ͬѧ�о��˼ס�����ͬѧ�ķ�����������һ�ַ����Ļ����������㷽���ⶨ��Wg��Ʒ�н���þ(Mg)�����������ķ����� �� ��5�����������Ҫ�����ǣ�����Һ����μ��� ��ֱ�����ɵij����պ��ܽ⣬�ټ��������� �� ��6��ij��Һ�к���Zn2+��Al3+��NH4+��SO42�������ӣ���������μ���NaOH��Һ�������ɳ��������ʵ��������NaOH��Һ�����ϵ��ͼ����ȷ����__________��
�鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |