
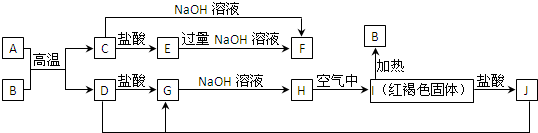
£Ø10·Ö£©A”«HÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£¬ĘäÖŠAŹĒŃĪ£¬B”¢C”¢D”¢E”¢FŌŚ³£ĪĀ³£Ń¹ĻĀ¾łŹĒĘųĢ¬ĪļÖŹ£¬·“Ó¦¢Ü”¢¢Ż¾łŌŚČÜŅŗÖŠ½ųŠŠ£¬×Ŗ»ÆÖŠ·“Ó¦Ģõ¼žĀŌČ„”£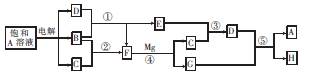
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĪļÖŹAµÄ»ÆѧŹ½ĪŖ £¬µē½āŹ±Ņõ¼«·“Ó¦Ź½ĪŖ .
£Ø2£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©·“Ó¦¢ŻµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø4£©Ć¾ÓėĀĮÄÜŠĪ³É¶ąÖÖŗĻ½š£¬ĪŖĮĖČ·¶ØijŗĻ½šŃłĘ·µÄ³É·Ö£¬Š”Ć÷Ķ¬Ń§Éč¼ĘČēĶ¼ĖłŹ¾µÄŹµŃé²½ Öč£¬Č·¶ØŗĻ½š×é³É”£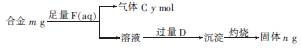
¢ŁÄÜČ·¶ØŗĻ½š×é³ÉµÄŹż¾Ż×éÓŠ £ØĢī×ÖÄø£©”£
a£®m”¢n b£®m”¢y c£®n”¢y
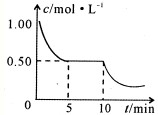
¢ŚČōŗĻ½šÖŠMgµÄĪļÖŹµÄĮæ·ÖŹżĪŖx£¬½šŹō×ÜĪļÖŹµÄĮæĪŖ7 mol£¬ŹŌŌŚĶ¼ÖŠ×÷³öyĖęx±ä»ÆµÄĒśĻß”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
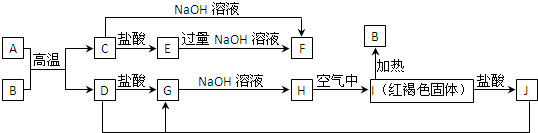
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø13·Ö£©ĻĀĮŠĶ¼ÖŠŹĒ֊ѧ»ÆѧµÄ³£¼ūĪļÖŹ£¬ĘäÖŠE”¢F”¢G”¢Z”¢WŹĒµ„ÖŹ£¬BµÄ»ÆѧŹ½ŹĒE2F3”£ĖüĆĒÓŠČēĻĀĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£ŗ
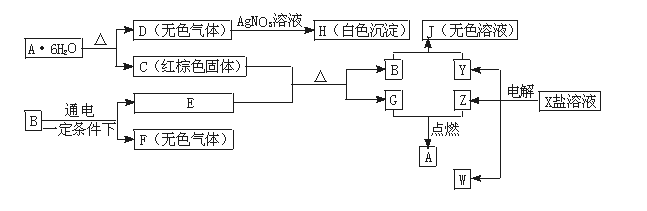
£Ø1£©F”¢HµÄ»ÆѧŹ½·Ö±šŹĒ_________________________ ”¢ _______________________”£
£Ø2£©C+E·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ______________________________________ ”£øĆ·“Ó¦µÄÓĆĶ¾Ö®Ņ»ŹĒ ”£
£Ø3£©Š“³öA”¤6H2O×Ŗ±äĪŖCŗĶDµÄ»Æѧ·½³ĢŹ½____________________________”£
£Ø4£©Š“³öµē½āXČÜŅŗµÄĄė×Ó·½³ĢŹ½_____________________________”£
£Ø5£©Š“³öBŗĶYČÜŅŗ·“Ӧɜ³ÉJµÄ»Æѧ·½³ĢŹ½_________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ź”¼ĆÄžŹŠĪ¢É½Ņ»ÖŠø߶ž£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com