
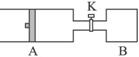
£Ø8·Ö£©ČēĻĀĶ¼ĖłŹ¾£¬ĻņAÖŠ³äČė1mol XŗĶ1mol Y£¬ĻņBÖŠ³äČė2mol XŗĶ2mol Y£¬ĘšŹ¼Ź±A”¢BµÄĢå»ż¾łĪŖa L£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢Éś·“Ó¦£ŗ
X(g)+Y(g) ?2Z(g)+W(g)£ØÕż·“Ó¦ĪŖ·ÅČČ·“Ó¦£©“ļµ½Ę½ŗāŹ±AČŻĘ÷µÄĢå»żĪŖ1.2a L”£
(1) AÖŠXµÄ×Ŗ»ÆĀŹ¦ĮA=
(2) A”¢BÖŠXµÄ×Ŗ»ÆĀŹ¦ĮA ¦ĮB£ØĢī >”¢< »ņ = £©
(3) “ņæŖK£¬Ņ»¶ĪŹ±¼äÓÖ“ļµ½Ę½ŗāŹ±AµÄĢå»żĪŖ L£ØĮ¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£©
(4) ŌŚ(3)“ļµ½Ę½ŗāŗó£¬Ķ¬Ź±µČ·łÉżøßA”¢BµÄĪĀ¶Č£¬“ļµ½ŠĀĘ½ŗāŗó£¬AµÄĢå»ż £ØĢī±ä“󔢱䊔»ņ²»±ä£©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)AÖŠXµÄ×Ŗ»ÆĀŹ¦Į(A)=_______________”£
(2)A”¢BÖŠX×Ŗ»ÆĀŹµÄ¹ŲĻµ£ŗ¦Į(A)_____________¦Į(B)(Ģī”°£¾”±”°=”±»ņ”°£¼”±)”£
(3)“ņæŖK£¬Ņ»¶ĪŹ±¼äÓÖ“ļĘ½ŗāŹ±£¬AµÄĢå»żĪŖ____________L(Į¬ĶعÜÖŠĘųĢåĢå»ż²»¼Ę)”£
(4)ŌŚ(3)“ļĘ½ŗāŗó£¬Ķ¬Ź±µČ·łÉżøßA”¢BµÄĪĀ¶Č£¬“ļŠĀĘ½ŗāŗóAµÄĢå»ż__________(Ģī”°±ä“ó”±”°²»±ä”±»ņ”°±äŠ””±)£¬ĘäĄķÓÉŹĒ________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø8·Ö£©ČēĻĀĶ¼ĖłŹ¾£¬ĻņAÖŠ³äČė1mol XŗĶ1mol Y£¬ĻņBÖŠ³äČė2mol XŗĶ2mol Y£¬ĘšŹ¼Ź±A”¢BµÄĢå»ż¾łĪŖa L£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢Éś·“Ó¦£ŗ
X(g)+Y(g) ⇌2Z(g)+W(g)£ØÕż·“Ó¦ĪŖ·ÅČČ·“Ó¦£©“ļµ½Ę½ŗāŹ±AČŻĘ÷µÄĢå»żĪŖ1.2a L”£
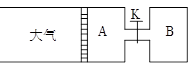
(1) AÖŠXµÄ×Ŗ»ÆĀŹ¦ĮA=
(2) A”¢BÖŠXµÄ×Ŗ»ÆĀŹ¦ĮA ¦ĮB£ØĢī >”¢< »ņ = £©
(3) “ņæŖK£¬Ņ»¶ĪŹ±¼äÓÖ“ļµ½Ę½ŗāŹ±AµÄĢå»żĪŖ L£ØĮ¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£©
(4) ŌŚ(3)“ļµ½Ę½ŗāŗó£¬Ķ¬Ź±µČ·łÉżøßA”¢BµÄĪĀ¶Č£¬“ļµ½ŠĀĘ½ŗāŗó£¬AµÄĢå»ż £ØĢī±ä“󔢱䊔»ņ²»±ä£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓ±±Ź”øßŅ»ĻĀѧʌȿµ÷æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©ČēĻĀĶ¼ĖłŹ¾£¬ĻņAÖŠ³äČė1mol XŗĶ1mol Y£¬ĻņBÖŠ³äČė2mol XŗĶ2mol Y£¬ĘšŹ¼Ź±A”¢BµÄĢå»ż¾łĪŖa L£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢Éś·“Ó¦£ŗ
X(g)+Y(g) ⇌2Z(g)+W(g)£ØÕż·“Ó¦ĪŖ·ÅČČ·“Ó¦£©“ļµ½Ę½ŗāŹ±AČŻĘ÷µÄĢå»żĪŖ1.2a L”£
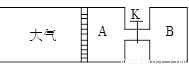
(1) AÖŠXµÄ×Ŗ»ÆĀŹ¦ĮA=
(2) A”¢BÖŠXµÄ×Ŗ»ÆĀŹ¦ĮA ¦ĮB£ØĢī >”¢< »ņ = £©
(3) “ņæŖK£¬Ņ»¶ĪŹ±¼äÓÖ“ļµ½Ę½ŗāŹ±AµÄĢå»żĪŖ L£ØĮ¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£©
(4) ŌŚ(3)“ļµ½Ę½ŗāŗó£¬Ķ¬Ź±µČ·łÉżøßA”¢BµÄĪĀ¶Č£¬“ļµ½ŠĀĘ½ŗāŗó£¬AµÄĢå»ż £ØĢī±ä“󔢱䊔»ņ²»±ä£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĻĀĶ¼ĖłŹ¾£¬ĻņAÖŠ³äČė1 mol XŗĶ1 mol Y£¬ĻņBÖŠ³äČė2 mol XŗĶ2 mol Y£¬ĘšŹ¼Ź±£¬VA=VB=a L”£ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀŹö·“Ó¦£ŗ
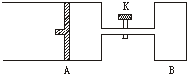
X+Y![]() 2Z+W ¦¤H£¼0(X”¢Y”¢Z”¢W¾łĪŖĘųĢå)£¬“ļµ½Ę½ŗāŹ±£¬VA=1.2a L”£
2Z+W ¦¤H£¼0(X”¢Y”¢Z”¢W¾łĪŖĘųĢå)£¬“ļµ½Ę½ŗāŹ±£¬VA=1.2a L”£
ŹŌ»Ų“š£ŗ
(1)AÖŠXµÄ×Ŗ»ÆĀŹaA=________£»
(2)A”¢BÖŠX×Ŗ»ÆĀŹµÄ¹ŲĻµ£ŗaA_________aB(Ģī”°£¾”±”°=”±»ņ”°£¼”±)£»
(3)“ņæŖK£¬Ņ»¶ĪŹ±¼äŗóÓÖ“ļĘ½ŗāŹ±£¬AµÄĢå»żĪŖ________L(Į¬ĶعÜÖŠĘųĢåĢå»ż²»¼Ę)£»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com