

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
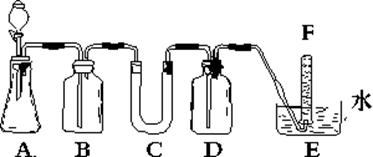
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
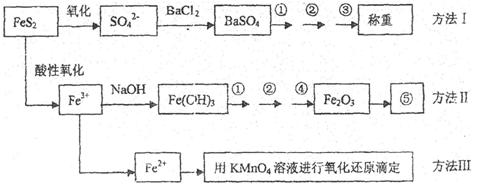
| A���������� | B������ʱ���� |
| C������ʱ���ʺ�����λ�÷��ˣ���Ҫ���룩 | D������ƿ�ô�װҺ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������ �����������ۣ���������ȩ�� �����������ۣ���������ȩ�� |
B�� ij±�������� ij±��������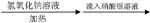 ���յij������ǰ�ɫ�� ���յij������ǰ�ɫ�����ۣ���±�����в�����ԭ�� |
C��ij ��Һ ��Һ ð�Ű��� ð�Ű��� �����������̣� �����������̣����ۣ�����ҺΪŨ���� |
D����ɫ��Һ �ʻ�ɫ�����ۣ�����Һһ��������Ԫ�� �ʻ�ɫ�����ۣ�����Һһ��������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������0.1mol/L������500mL��Ӧѡ�õ������н�ͷ�ιܡ��ձ�������������ƽ��500mL����ƿ |
| B�����Ʊ�Fe(OH)3���壬��ʢ�з�ˮ���ձ��еμ�FeCl3������Һ����ʱ����� |
| C��Ϊ����KCl��AlCl3��MgCl2��Һ���ֱ���������Һ�еμ�NaOH��Һ������ |
| D���ⶨ����ͭ�����нᾧˮ����, ���������о�����ȫʧˮ�����ڿ�������ȴ���ٳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ����֤����ȫȼ�պ����ֻ��H2O��CO2 |
| B���ⶨ����������������������ȫȼ�պ�����CO2��H2O������ |
| C���ⶨ��ȫȼ��ʱ�����л��������ɵ�CO2��H2O�����ʵ���֮�� |
| D��ֻ�вⶨ��ȼ�ղ�����H2O��CO2���ʵ����ı�ֵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ƺ�þ�ֱ�����ˮ��Ӧ���ж��ƺ�þ������ǿ�� |
| B����MgCl2��AlCl3��Һ�зֱ��������İ�ˮ���ж�þ�����Ľ�����ǿ�� |
| C����������Һ��ͨ��CO2������ɫ�������ж�̼������������ǿ�� |
| D��Br2��I2�ֱ���������H2��Ӧ���ж������ķǽ�����ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
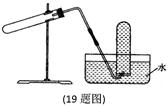
| A������CaCO3��CO2 |
| B����Cu��ϡHNO3��Ӧ��NO |
| C����NH4Cl��ŨNaOH��Һ��Ӧ��NH3 |
| D����NaCl��ŨH2SO4��Ӧ��HC1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com