
”¾ĢāÄæ”æŅĄ¾ŻĶ¼ÖŠµŖŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄ×Ŗ»Æ¹ŲĻµ£¬»Ų“šĪŹĢā£ŗ
£Ø1£©ŹµŃéŹŅ³£ÓĆNH4ClÓėCa(OH)2ÖĘČ”°±Ęų£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________”£
£Ø2£©ČōŅŖŹÕ¼ÆŅ»Ęæ°±Ęų£¬ĒėŌŚĶ¼ŠéæņÄŚ»³öĮ¬½ÓĶ¼_____”£
£Ø3£©¹¤ŅµÉĻŅŌNH3”¢æÕĘų”¢Ė®ĪŖŌĮĻÉś²śĻõĖįµÄ¹¤ŅÕĮ÷³Ģ¼ņĶ¼ČēĻĀĖłŹ¾£ŗ
![]()
Š“³öNH3”śNOµÄ»Æѧ·½³ĢŹ½__________________________________”£
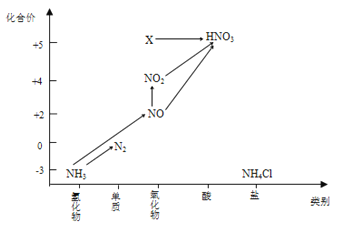
£Ø4£©Ķ¼ÖŠ£¬XµÄ»ÆѧŹ½ĪŖ_______£¬½«X×Ŗ»ÆĪŖHNO3ŹōÓŚ______________·“Ó¦£ØĢī”°Ńõ»Æ»¹Ō”±»ņ”°·ĒŃõ»Æ»¹Ō”±£©·“Ó¦”£
£Ø5£©ČōŅŖ½«NH3”śN2£¬“ÓŌĄķÉĻæ“£¬ĻĀĮŠŹŌ¼ĮæÉŠŠµÄŹĒ________£ØĢīŠņŗÅ£©”£
A£®O2 B£®Na C£®NH4Cl D£®NO2
”¾“š°ø”æ2NH4Cl£«Ca(OH)2![]() 2NH3”ü£«CaCl2£«2H2O
2NH3”ü£«CaCl2£«2H2O  4NH3£«5O2
4NH3£«5O2![]() 4NO£«6H2O N2O5 ·ĒŃõ»Æ»¹Ō AD
4NO£«6H2O N2O5 ·ĒŃõ»Æ»¹Ō AD
”¾½āĪö”æ
£Ø1£©ŹµŃéŹŅ³£ÓĆNH4ClÓėCa(OH)2ÖĘČ”°±Ęų£¬Éś³ÉĀČ»ÆøĘ”¢°±ĘųŗĶĖ®£»
£Ø2£©°±Ęų¼«Ņ×ČÜÓŚĖ®£¬±ČæÕĘųĒį£¬ŹÕ¼Æ·½·ØÖ»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£¬µ¼Ęų¹ÜĪ»ÖƶĢ½ų³¤³ö£»
£Ø3£©°±ĘųµÄ“ß»ÆŃõ»Æ£ŗ4NH3+5O2![]() 4NO+6H2O£»¢ŚNO”śNO2ŹµŃéĻÖĻóŹĒĪŽÉ«ĘųĢå±ä»ÆĪŖŗģ×ŲÉ«ĘųĢ壻¢Ū¶žŃõ»ÆµŖŗĶĖ®·“Ӧɜ³ÉŅ»Ńõ»ÆµŖŗĶĻõĖį£»
4NO+6H2O£»¢ŚNO”śNO2ŹµŃéĻÖĻóŹĒĪŽÉ«ĘųĢå±ä»ÆĪŖŗģ×ŲÉ«ĘųĢ壻¢Ū¶žŃõ»ÆµŖŗĶĖ®·“Ӧɜ³ÉŅ»Ńõ»ÆµŖŗĶĻõĖį£»
£Ø4£©Ķ¼1·ÖĪöæÉÖŖX»ÆŗĻ¼ŪÓėĻõĖįĻąĶ¬ĪŖ+5¼Ū£¬ĪļÖŹĄąŠĶĪŖŃõ»ÆĪļ£¬X»ÆѧŹ½ĪŖ£ŗN2O5£¬“ÓĪļÖŹŠŌÖŹÉĻæ“£¬XŹōÓŚĖįŠŌŃõ»ÆĪļN2O5+2H2O=2HNO3£¬×Ŗ»ÆĪŖHNO3ŹōÓŚ·ĒŃõ»Æ»¹Ō·“Ó¦£»
£Ø7£©ČōŅŖ½«NH3”śN2,“ÓŌĄķÉĻæ“£¬¾ßÓŠŃõ»ÆŠŌµÄŹŌ¼ĮæÉŠŠ£»
£Ø1£©ŹµŃéŹŅ³£ÓĆNH4ClÓėCa(OH)2ÖĘČ”°±Ęų£¬Éś³ÉĀČ»ÆøĘ”¢°±ĘųŗĶĖ®£¬»Æѧ·½³ĢŹ½ĪŖ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3+2H2O£»
CaCl2+2NH3+2H2O£»
ÕżČ·“š°ø£ŗ2NH4Cl£«Ca(OH)2![]() 2NH3”ü£«CaCl2£«2H2O
2NH3”ü£«CaCl2£«2H2O
£Ø2£©°±Ęų¼«Ņ×ČÜÓŚĖ®£¬±ČæÕĘųĒį£¬ŹÕ¼Æ·½·ØÖ»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£¬µ¼Ęų¹ÜĪ»ÖƶĢ½ų³¤³ö£» £»
£»
ÕżČ·“š°ø£ŗ
£Ø3£©°±ĘųµÄ“ß»ÆŃõ»Æ£ŗ4NH3+5O2![]() 4NO+6H2O£»¢ŚNO”śNO2ŹµŃéĻÖĻóŹĒĪŽÉ«ĘųĢå±ä»ÆĪŖŗģ×ŲÉ«ĘųĢ壻¢Ū¶žŃõ»ÆµŖŗĶĖ®·“Ӧɜ³ÉŅ»Ńõ»ÆµŖŗĶĻõĖį£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ3NO2+H2O=2HNO3+NO£»
4NO+6H2O£»¢ŚNO”śNO2ŹµŃéĻÖĻóŹĒĪŽÉ«ĘųĢå±ä»ÆĪŖŗģ×ŲÉ«ĘųĢ壻¢Ū¶žŃõ»ÆµŖŗĶĖ®·“Ӧɜ³ÉŅ»Ńõ»ÆµŖŗĶĻõĖį£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ3NO2+H2O=2HNO3+NO£»
ÕżČ·“š°ø£ŗ4NH3+5O2![]() 4NO+6H2O”£
4NO+6H2Oӣ
£Ø4£©Ķ¼1·ÖĪöæÉÖŖX»ÆŗĻ¼ŪÓėĻõĖįĻąĶ¬ĪŖ+5¼Ū£¬ĪļÖŹĄąŠĶĪŖŃõ»ÆĪļ£¬X»ÆѧŹ½ĪŖ£ŗN2O5£¬“ÓĪļÖŹŠŌÖŹÉĻæ“£¬XŹōÓŚĖįŠŌŃõ»ÆĪļN2O5+2H2O=2HNO3£¬×Ŗ»ÆĪŖHNO3ŹōÓŚ·ĒŃõ»Æ»¹Ō·“Ó¦£»
ÕżČ·“š°ø£ŗN2O5 ·ĒŃõ»Æ»¹Ō”£
£Ø7£©ČōŅŖ½«NH3”śN2,“ÓŌĄķÉĻæ“£¬¾ßÓŠŃõ»ÆŠŌµÄŹŌ¼ĮæÉŠŠ£»
A.O2¾ßÓŠŃõ»ÆŠŌ£¬¹ŹAÕżČ·£»
B.NaÖ»¾ßÓŠ»¹ŌŠŌ£¬¹ŹB“ķĪó£»
C.NH4ClÓė°±Ęų²»·“Ó¦£¬¹ŹC“ķĪó£»
D.NO2¾ßÓŠŃõ»ÆŠŌ£¬¹ŹDÕżČ·£»
ÕżČ·“š°ø£ŗAD


 Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø
Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©K2Cr2O7 + 14HCl= 2KCl + 2CrCl3 + 3Cl2”ü+ 7H2O £ØÓĆ”°µ„ĻßĒÅ”±±ķŹ¾µē×Ó×ŖŅʵķ½ĻņŗĶŹżÄ棩___,Ńõ»Æ²śĪļÓė»¹Ō²śĪļµÄĪļÖŹµÄĮæÖ®±ČĪŖ_____________”£
£Ø2£©______mol H2OÖŠ¹²ŗ¬ÓŠ9.03”Į1022øöŌ×Ó£¬ĘäÖŹĮæĪŖ_______”£
£Ø3£©ÅäĘ½ĻĀĮŠŃõ»Æ»¹Ō·“Ó¦·½³ĢŹ½£ŗ___KMnO4+___H2S+__H2SO4(Ļ”) ”Ŗ__MnSO4+__S”ż+__K2SO4+__H2O
£Ø4£©Cl2ŹĒŅ»ÖÖÓŠ¶¾ĘųĢ壬Čē¹ūŠ¹Ā©»įŌģ³ÉŃĻÖŲµÄ»·¾³ĪŪČ¾”£»Æ¹¤³§æÉÓĆÅØ°±Ė®Ą“¼ģŃéCl2ŹĒ·ńŠ¹Ā©£¬ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ3Cl2£ØĘų£©£«8NH3£ØĘų£©£½6NH4Cl£Ø¹Ģ£©£«N2£ØĘų£©£¬Čō·“Ó¦ÖŠĻūŗÄCl2 1.5 mol, Ōņ±»Ńõ»ÆµÄNH3ŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ______ L”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ³żŌÓĖłŃ”ÓƵďŌ¼Į¼°²Ł×÷·½·Ø¾łÕżČ·µÄŅ»×éŹĒ(ĄØŗÅÄŚĪŖŌÓÖŹ)
Ń”Ļī | “żĢį“æµÄĪļÖŹ | Ń”ÓƵďŌ¼Į | ²Ł×÷·½·Ø |
A | NaHCO3(Na2CO3) | ŹŹĮæŃĪĖį | Õō·¢½į¾§ |
B | CO2(CO) | O2 | µćČ¼ |
C | Mg(Al) | ĒāŃõ»ÆÄĘČÜŅŗ | ¹żĀĖ |
D | CO2(HCl) | ĒāŃõ»ÆÄĘČÜŅŗ | Ļ“Ęų |
A. A B. B C. C D. D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijČÜŅŗÓÉNa+”¢Cu2+”¢Ba2+”¢Fe3+”¢AlO![]() ”¢CO
ӢCO![]() ӢSO
”¢SO![]() ”¢ClÖŠµÄČōøÉÖÖĄė×Ó×é³É£¬Č”ŹŹĮæøĆČÜŅŗ½ųŠŠČēĻĀŹµŃé£ŗĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
”¢ClÖŠµÄČōøÉÖÖĄė×Ó×é³É£¬Č”ŹŹĮæøĆČÜŅŗ½ųŠŠČēĻĀŹµŃé£ŗĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A. ŌČÜŅŗÖŠŅ»¶ØÖ»“ęŌŚ![]() ”¢
Ӣ![]() Ӣ
”¢![]() ”¢Cl-ĖÄÖÖĄė×Ó
”¢Cl-ĖÄÖÖĄė×Ó
B. ĘųĢåAµÄ»ÆѧŹ½ŹĒCO2£¬Ęäµē×ÓŹ½ĪŖO::C::O
C. ŌČÜŅŗÖŠŅ»¶Ø²»“ęŌŚµÄĄė×ÓŹĒCu2+”¢Ba2+”¢Fe3+
D. Éś³É³ĮµķBµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAl3++3OH===Al(OH)3”ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ
A. ³£ĪĀ³£Ń¹ĻĀ£¬1.7 g°±ĘųÖŠŗ¬ÓŠµÄŌ×ÓŹżÄæĪŖ0.4NA
B. 50 mL 1 mol”¤L1 K2SO4ČÜŅŗÖŠŗ¬ÓŠµÄK+ŹżÄæĪŖ0.1NA
C. 5.6 gĢśÓė×ćĮæĻ”ĮņĖį·“Ó¦×ŖŅʵĵē×ÓŹżĪŖ0.3NA
D. ±ź×¼×“æöĻĀ£¬4.48 LµÄŃõĘųŗĶµŖĘųµÄ»ģŗĻĪļŗ¬ÓŠµÄ·Ö×ÓŹżÄæĪŖ0.2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»Æѧ¼ĘĮæŌŚ»ÆŃ§ÖŠÕ¼ÓŠÖŲŅŖµŲĪ»”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©0.3 mol NH3·Ö×ÓÖŠĖłŗ¬Ō×ÓŹżÓėŌ¼__________øöH2O·Ö×ÓÖŠĖłŗ¬Ō×ÓŹżĻąµČ”£
£Ø2£©V mLŗ¬a g Al3+µÄAl2(SO4)3ČÜŅŗÖŠĖłŗ¬SO42µÄĪļÖŹµÄĮæÅضČĪŖ_____ mol”¤L1”£
£Ø3£©ŌŚŅ»¶ØĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬1Ģå»żĘųĢåX2Óė3Ģå»żY2»ÆŗĻÉś³É2Ģå»żĘųĢ¬»ÆŗĻĪļ£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ_________”£
£Ø4£©½«ø÷0.3 molµÄÄĘ”¢Ć¾”¢ĀĮ·Ö±š·ÅČė100 mL 1 mol”¤L1µÄŃĪĖįÖŠ£¬Ķ¬ĪĀĶ¬Ń¹ĻĀ²śÉśĘųĢåµÄĢå»ż±ČĪŖ_____________”£
£Ø5£©ŠæÓėŗÜĻ”µÄĻõĖį·“Ӧɜ³ÉĻõĖįŠæ”¢ĻõĖįļ§ŗĶĖ®£¬µ±Éś³É1 molĻõĖįŠæŹ±£¬²Ī¼Ó·“Ó¦µÄĻõĖįµÄĪļÖŹµÄĮæĪŖ_________ mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪ¢ÉśĪļµē³ŲŹĒÖøŌŚĪ¢ÉśĪļµÄ×÷ÓĆĻĀ½«»ÆѧÄÜ×Ŗ»ÆĪŖµēÄܵÄ×°ÖĆ£¬Ę乤×÷ŌĄķČēĶ¼ĖłŹ¾”£ĻĀĮŠÓŠ¹ŲĪ¢ÉśĪļµē³ŲµÄĖµ·Ø“ķĪóµÄŹĒ ( )

A. Õż¼«·“Ó¦ÖŠÓŠCO2Éś³É
B. Ī¢ÉśĪļ“Ł½ųĮĖ·“Ó¦ÖŠµē×ÓµÄ×ŖŅĘ
C. ÖŹ×ÓĶعż½»»»Ä¤“Óøŗ¼«ĒųŅĘĻņÕż¼«Ēų
D. µē³Ų×Ü·“Ó¦ĪŖC6H12O6£«6O2===6CO2£«6H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潫0.2molMn02ŗĶ50mL12mol/LŃĪĖį»ģŗĻŗó¼ÓČČ£¬·“Ó¦ĶźČ«ŗóĻņĮōĻĀµÄČÜŅŗÖŠ¼ÓČė×ćĮæAgNO3ČÜŅŗ£¬Éś³ÉAgCl³ĮµķĪļÖŹµÄĮæĪŖ£Ø²»æ¼ĀĒŃĪĖįµÄ»Ó·¢£©£Ø £©
A. µČÓŚ0.3mol B. Š”ÓŚ0.3mol C. “óÓŚ 0.3mol£¬Š”ÓŚ 0.6mol D. µČÓŚ 0.6mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪŅ¹ś¹Å“śµÄ¼¼ŹõÓ¦ÓĆÖŠ£¬Ę乤×÷ŌĄķ²»Éę¼°»Æѧ·“Ó¦µÄŹĒ
A.ɳĄļĢŌ½šB.ĮøŹ³Äš¾ĘC.»šŅ©Ź¹ÓĆD.ĢśµÄŅ±Į¶
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com