
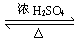 CH3OOCCH��CHCOOCH3 + 2H2O
CH3OOCCH��CHCOOCH3 + 2H2O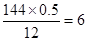 ������ˮ�����ɼ״���������A��ֻ��1����������B�ķ���ʽ����C5H5O5,���Լ����Dz��ܳ����ģ�����A�к���2��������ˮ������2���Ӽ״������B�ķ���ʽΪC4H4O4,����ΪB��û��֧��������B�Ľṹ��ʽΪHOOCCH��CHCOOH����A�Ľṹ��ʽΪCH3OOCCH��CHCOOCH3��ͨ���ı��Ȼ���λ�ü��õ���B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2��C(COOH)2��
������ˮ�����ɼ״���������A��ֻ��1����������B�ķ���ʽ����C5H5O5,���Լ����Dz��ܳ����ģ�����A�к���2��������ˮ������2���Ӽ״������B�ķ���ʽΪC4H4O4,����ΪB��û��֧��������B�Ľṹ��ʽΪHOOCCH��CHCOOH����A�Ľṹ��ʽΪCH3OOCCH��CHCOOCH3��ͨ���ı��Ȼ���λ�ü��õ���B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2��C(COOH)2��

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͬϵ��Ļ�ѧ�������� |
| B����ϩ�ͻ�������ͬ���칹�� |
| C����Է���������ͬ�ļ��ֻ����ﻥ��Ϊͬ���칹�� |
| D���������������ɸ�CH2ԭ���ŵ��л��ﲻһ����ͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2:1 | B��3:1 | C��1:3 | D��1:1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
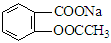 ת��Ϊ
ת��Ϊ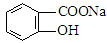 ����Ϊ ( )
����Ϊ ( )| A����ϡH2SO4���Ⱥ�������Na2CO3 |
| B����ϡH2SO4���Ⱥ�������NaOH |
| C��������NaOH��Һ���Ⱥ���ͨ��CO2 |
| D��������NaOH��Һ���Ⱥ��ټ���ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼Ԫ��ת��ΪNaCN | B����Ԫ��ת��Ϊ�������� |
| C����Ԫ��ת��Ϊ�廯�� | D����Ԫ��ת��Ϊ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com