
| 0.01xЁС2 |
| 3 |
| 0.002mol |
| 2 |
| 0.02x |
| 3 |
| 10.2g |
| 17g/mol |
|
|
| 8.96L |
| 22.4L/mol |
|
|
| 0.1mol |
| 0.1L |
| 1 |
| 2 |
| 1 |
| 2 |



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2012НьЩНЖЋЪЁлВГЧжАвЕИпжаИпШ§3дТдТПМРэзлВПЗжЃЈДјНтЮіЃЉ ЬтаЭЃКЬюПеЬт
ЃЈ 12ЗжЃЉюбЃЈTiЃЉБЛГЦЮЊМЬЬњЁЂТСжЎКѓЕФЕкШ§Н№ЪєЃЌЖлюбКЭвдюбЮЊжїЕФКЯН№ЪЧаТаЭЕФНсЙЙВФСЯЃЌжївЊгУгкКНЬьЙЄвЕКЭКНКЃЙЄвЕЃЌЯТСаЪЧгаЙиюбЕФвБСЖМАгІгУЕФЮЪЬтЁЃ
ЃЈ1ЃЉН№ЪєюбвБСЖЙ§ГЬжаЦфжавЛВНЗДгІЪЧНЋдСЯН№КьЪЏзЊЛЏЃКTiO2ЃЈН№КьЪЏЃЉ+2C+2Cl2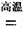 TiCl4+2CO
TiCl4+2CO
вбжЊЃКCЃЈSЃЉ+O2ЃЈgЃЉЃНCO2ЃЈgЃЉ  HЃНЃ393ЃЎ5 kJЁЄmol-1
HЃНЃ393ЃЎ5 kJЁЄmol-1
2COЃЈgЃЉЃЋO2ЃЈgЃЉЃН2CO2ЃЈgЃЉ  HЃН-566 kJЁЄmol-1
HЃН-566 kJЁЄmol-1
TiO2ЃЈsЃЉ+2Cl2ЃЈgЃЉЃНTiCl4ЃЈsЃЉ+O2ЃЈgЃЉ  HЃН+141 kJЁЄmol-1
HЃН+141 kJЁЄmol-1
дђTiO2ЃЈgЃЉ+2Cl2ЃЈgЃЉ+2CЃЈsЃЉЃНTiCl4ЃЈsЃЉ+2COЃЈgЃЉЕФ HЃН ЃЌ
HЃН ЃЌ
ЃЈ2ЃЉФЦШШЛЙдЗЈЪЧвБСЖН№ЪєюбЕФЗНЗЈжЎвЛЃЌжївЊЗДгІдРэЮЊЃК4Na+TiCl4 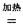 4NaCl+TiЃЌИУЗДгІВЛФмдкЫЎШмвКжаНјааЃЌвЛЪЧвђЮЊTiCl4ЛсЧПСвЫЎНтЩњГЩTiO2ЃЌСэвЛдвђ ЃЈгУЪЪЕБЛЏбЇЗНГЬЪНИЈвдБивЊЕФЮФзжЫЕУїЃЉЁЃ
4NaCl+TiЃЌИУЗДгІВЛФмдкЫЎШмвКжаНјааЃЌвЛЪЧвђЮЊTiCl4ЛсЧПСвЫЎНтЩњГЩTiO2ЃЌСэвЛдвђ ЃЈгУЪЪЕБЛЏбЇЗНГЬЪНИЈвдБивЊЕФЮФзжЫЕУїЃЉЁЃ
ЃЈ3ЃЉУОЛЙдЗЈвВЪЧвБСЖН№ЪєюбЕФГЃгУЗНЗЈЃЌЦфжївЊЗДгІдРэШчЯТЃК
MgCl2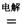 Mg+Cl2 TiCl4+2Mg
Mg+Cl2 TiCl4+2Mg 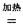 2MgCl2+Ti
2MgCl2+Ti
ДгКЃЫЎжаЬсШЁMgCl2ЪБЃЌЯШдкКЃЫЎжаМгШыЪьЪЏЛвЃЌГСЕэГіMgЃЈOHЃЉ2ЃЌаДГіMgЃЈOHЃЉ2ШмЖШЛ§БэДяЪНЃК
ПЩМгШыЪЪЕБЙ§СПЕФЪьЪЏЛвЃЌДгMgЃЈOHЃЉ2ШмНтЦНКтНЧЖШНтЪЭЦфдвђ
ЃЈ4ЃЉTiCl4гыLiOHдкЫЎШмвКжавЛЖЈЬѕМўЯТПЩЗДгІЩњГЩLi4Ti5O12ЃЈюбЫсяЎЃЉЃЌLi4Ti5O12ПЩгыLiMn2O4ЃЈУЬЫсяЎЃЉЕШе§МЋВФСЯзщГЩРэРызгЖўДЮЕчГиЃЌЙЄзїЪБLi+ дкЕчГиФкЖЈЯђвЦЖЏЃЌЦфЕчГиЗДгІЮЊЃК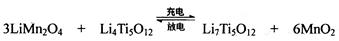 ЃЌЪЙгУЪБЯШГфЕчЃЌаДГіЦфГфЕчЪНЕФбєМЋЗДгІ ЃЌЗХЕчЪБLi+ЕФвЦЖЏЗНЯђ ЁЃ
ЃЌЪЙгУЪБЯШГфЕчЃЌаДГіЦфГфЕчЪНЕФбєМЋЗДгІ ЃЌЗХЕчЪБLi+ЕФвЦЖЏЗНЯђ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2011-2012бЇФъЩНЖЋЪЁИпШ§ЕкЫФДЮеяЖЯПМЪдРэПЦзлКЯЪдОэЃЈЛЏбЇВПЗжЃЉ ЬтаЭЃКЬюПеЬт
ЃЈ 12ЗжЃЉюбЃЈTiЃЉБЛГЦЮЊМЬЬњЁЂТСжЎКѓЕФЕкШ§Н№ЪєЃЌЖлюбКЭвдюбЮЊжїЕФКЯН№ЪЧаТаЭЕФНсЙЙВФСЯЃЌжївЊгУгкКНЬьЙЄвЕКЭКНКЃЙЄвЕЃЌЯТСаЪЧгаЙиюбЕФвБСЖМАгІгУЕФЮЪЬтЁЃ
ЃЈ1ЃЉН№ЪєюбвБСЖЙ§ГЬжаЦфжавЛВНЗДгІЪЧНЋдСЯН№КьЪЏзЊЛЏЃКTiO2ЃЈН№КьЪЏЃЉ+2C+2Cl2 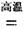 TiCl4+2CO
TiCl4+2CO
вбжЊЃКCЃЈSЃЉ+O2ЃЈgЃЉЃНCO2ЃЈgЃЉ 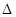 HЃНЃ393ЃЎ5 kJЁЄmol-1
HЃНЃ393ЃЎ5 kJЁЄmol-1
2COЃЈgЃЉЃЋO2ЃЈgЃЉЃН2CO2ЃЈgЃЉ 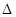 HЃН-566 kJЁЄmol-1
HЃН-566 kJЁЄmol-1
TiO2ЃЈsЃЉ+2Cl2ЃЈgЃЉЃНTiCl4ЃЈsЃЉ+O2ЃЈgЃЉ 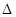 HЃН+141 kJЁЄmol-1
HЃН+141 kJЁЄmol-1
дђTiO2ЃЈgЃЉ+2Cl2ЃЈgЃЉ+2CЃЈsЃЉЃНTiCl4ЃЈsЃЉ+2COЃЈgЃЉЕФ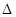 HЃН
ЃЌ
HЃН
ЃЌ
ЃЈ2ЃЉФЦШШЛЙдЗЈЪЧвБСЖН№ЪєюбЕФЗНЗЈжЎвЛЃЌжївЊЗДгІдРэЮЊЃК4Na+TiCl4 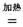 4NaCl+TiЃЌИУЗДгІВЛФмдкЫЎШмвКжаНјааЃЌвЛЪЧвђЮЊTiCl4ЛсЧПСвЫЎНтЩњГЩTiO2ЃЌСэвЛдвђ
ЃЈгУЪЪЕБЛЏбЇЗНГЬЪНИЈвдБивЊЕФЮФзжЫЕУїЃЉЁЃ
4NaCl+TiЃЌИУЗДгІВЛФмдкЫЎШмвКжаНјааЃЌвЛЪЧвђЮЊTiCl4ЛсЧПСвЫЎНтЩњГЩTiO2ЃЌСэвЛдвђ
ЃЈгУЪЪЕБЛЏбЇЗНГЬЪНИЈвдБивЊЕФЮФзжЫЕУїЃЉЁЃ
ЃЈ3ЃЉУОЛЙдЗЈвВЪЧвБСЖН№ЪєюбЕФГЃгУЗНЗЈЃЌЦфжївЊЗДгІдРэШчЯТЃК
MgCl2 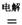 Mg+Cl2
TiCl4+2Mg
Mg+Cl2
TiCl4+2Mg 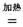 2MgCl2+Ti
2MgCl2+Ti
ДгКЃЫЎжаЬсШЁMgCl2ЪБЃЌЯШдкКЃЫЎжаМгШыЪьЪЏЛвЃЌГСЕэГіMgЃЈOHЃЉ2ЃЌаДГіMgЃЈOHЃЉ2ШмЖШЛ§БэДяЪНЃК
ПЩМгШыЪЪЕБЙ§СПЕФЪьЪЏЛвЃЌДгMgЃЈOHЃЉ2ШмНтЦНКтНЧЖШНтЪЭЦфдвђ
ЃЈ4ЃЉTiCl4гыLiOHдкЫЎШмвКжавЛЖЈЬѕМўЯТПЩЗДгІЩњГЩLi4Ti5O12ЃЈюбЫсяЎЃЉЃЌLi4Ti5O12ПЩгыLiMn2O4ЃЈУЬЫсяЎЃЉЕШе§МЋВФСЯзщГЩРэРызгЖўДЮЕчГиЃЌЙЄзїЪБLi+ дкЕчГиФкЖЈЯђвЦЖЏЃЌЦфЕчГиЗДгІЮЊЃК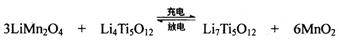 ЃЌЪЙгУЪБЯШГфЕчЃЌаДГіЦфГфЕчЪНЕФбєМЋЗДгІ ЃЌЗХЕчЪБLi+ЕФвЦЖЏЗНЯђ
ЁЃ
ЃЌЪЙгУЪБЯШГфЕчЃЌаДГіЦфГфЕчЪНЕФбєМЋЗДгІ ЃЌЗХЕчЪБLi+ЕФвЦЖЏЗНЯђ
ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
(1)ЖдвЛаЉВЛЛюЦУН№ЪєЃЌПЩвджБНггУ_____________ЕФЗНЗЈДгЦфЛЏКЯЮяжаЛЙдГіРДЁЃР§ШчЙЏКЭвјЁЃЦфЛЏбЇЗНГЬЪНШчЯТЃК___________________________________________________________ЁЃ
(2)ЖдгквЛаЉЗЧГЃЛюЦУЕФН№ЪєЃЌВЩгУвЛАуЕФЛЙдМСКмФбНЋЫќУЧЛЙдГіРДЃЌЙЄвЕЩЯГЃВЩгУ_____________вБСЖЁЃР§ШчФЦЁЂУОЁЂТСЕШН№ЪєЁЃЦфвБСЖЪБЗЂЩњЕФЛЏбЇЗНГЬЪНЗжБ№ЮЊ_____________ЁЂ_____________ЁЂ_____________ЁЃ
(3)ЖдгкН№ЪєЛюЖЏЫГађБэжаZnЁЋCuЖЮЕФН№ЪєЖМЪЧдкИпЮТЯТВЩгУ_____________ЃЌГЃгУЕФЛЙдМСга_____________ЁЂ_____________ЁЂ_____________ЁЂ_____________ЕШЁЃЖдгквЛаЉИпШлЕуЕФН№ЪєШчЬњЁЂИѕЁЂУЬЁЂЮйЕШПЩвдгУ_____________вБСЖЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com