
Ϊ���о���ѧ��ӦX��Y===Z�������仯�����ijͬѧ�������ͼ��ʾװ�á�����ʢ��X���Թ��еμ��Լ�Yʱ������U�ι��м״�Һ���½��Ҵ�Һ���������ɴ�˵��(����)

�ٷ�ӦΪ���ȷ�Ӧ
����������������ȷ�Ӧ�����������
�������еĻ�ѧ��ͨ����ѧ��Ӧת���������ͷų���
�ܷ�Ӧ�ﻯѧ���������յ��������������ﻯѧ���γɷų�������
A���٢� B���٢�
C���٢ڢ� D���ڢۢ�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ֶ�����Ԫ�������ڱ��е����λ��������ʾ������ZԪ��ԭ�Ӻ��������������������������3����
| X | Y | |
| Z | W |
��ش��������⣺
(1)Ԫ��Zλ�����ڱ��е�________����________�壻
(2)��ЩԪ�ص��⻯���У�ˮ��Һ������ǿ����________(д��ѧʽ)��
(3)XW2�Ļ�ѧʽΪ________________��
(4)Y�����������Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ĸ����Ҫ���������β��ϣ�������Ҫ�ɷ�ΪK2Al6Si6(OH)nO18����nΪ(����)
A��4 B��6 C��8 D��10
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӻ�ˮ����ȡ��Ĺ����У������·�Ӧ��5NaBr��NaBrO3��3H2SO4===3Br2��3Na2SO4��3H2O����������Ӧ��ԭ���������Ƶķ�Ӧ��(����)
A��2NaBr��Cl2===Br2��2NaCl
B��2KI��Br2===I2��2KBr
C��2H2S��SO2===3S����2H2O
D��Cl2��H2O===HCl��HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ�����������ͼ��

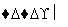

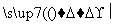

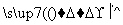
(1)ָ����ȡ��Ĺ������йص�ʵ��������ƣ�
��____________����____________��
д��ʵ������йط�Ӧ�����ӷ���ʽ��____________________________________��
(2)��ȡ��Ĺ����У��ɹ�ѡ����й��Լ���____��
A���ƾ� B�����Ȼ�̼
C������ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�����Թܷ���ʢ��25 ��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ���ٵ���5 mL���ᣬ�Իش��������⡣
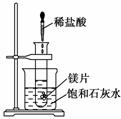
(1)ʵ���й۲쵽��������_________________________________________________
________________________________________________________________________��
(2)�������������ԭ����__________________________________________________
________________________________________________________________________
________________________________________________________________________��
(3)д���йط�Ӧ�����ӷ���ʽ��___________________________________________��
(4)��ʵ����֪��MgCl2��Һ��H2��������________(����ڡ�����С�ڡ����ڡ�)þƬ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���20 mL 0��2 mol/L H2A��Һ�еμ�0��2 mol/L NaOH��Һ���й��������ʵ����仯����ͼ(���Т����H2A�������HA���������A2��)������ͼʾ�жϣ�����˵����ȷ����
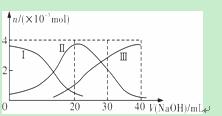
A��H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A===H����HA����HA��H����A2��
B����V(NaOH)��20 mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c(Na��)>c(HA��)>c(H��)>c(A2��)>c(OH��)
C���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ��
D����V(NaOH)��30 mLʱ����Һ�д������¹�ϵ��2c(H��)��c(HA��)��2c(H2A)��c(A2��)��2c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ϱ�������Ŀǰ�������н�20���˻���ȱ����ƶѪ��Ӱ�����˵����彡�������������и��Ͷ�ͯΣ���������ء����ҹ������������ˡ����Ͳ������̡������������ָ
A����Ԫ�� B�������� C�������� D������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڽ����˵���У���ȷ����(����)
A����������Һ�����Բ�ͬ���������������ʽ�״
B�����岻�ȶ�����ֹ�����ײ�������
C����Fe(OH)3������й��ˣ����õ�����Һ��ԭ�����������Dz�ͬ��
D�������ǽ��壬��Ϊ�����еķ�ɢ����ֱ����1��100 nm֮��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com