
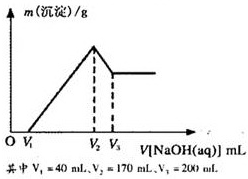
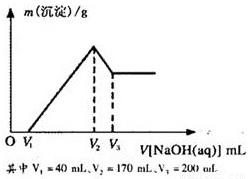
 Ć¾ĀĮŗĻ½šŹĒŃĢ»ØÉś²ś¹ż³ĢÖŠµÄÖŲŅŖŌĮĻ£¬Ķ¬Ź±Ņ²æÉ×÷ĪŖ°×¹ā¼ĮŗĶÕÕĆ÷¼Į£®ĪŖĮĖĢ½¾æĆ¾ĀĮŗĻ½šÖŠø÷³É·ÖµÄŗ¬Į棬æµæµĶ¬Ń§½«Ņ»¶ØÖŹĮæµÄĆ¾ĀĮŗĻ½šĶ¶Čėµ½850mL”¢Ņ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖįÖŠ£¬ŗĻ½šČ«²æČܽā£¬Č»ŗóÓÖµĪ¼Ó5mol/LµÄNaOHČÜŅŗ£®ČōŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķÖŹĮæ£Øm£©Ėę¼ÓČėNaOHČÜŅŗµÄĢå»ż£ØV£©µÄ±ä»ÆČēĶ¼ĖłŹ¾£®
Ć¾ĀĮŗĻ½šŹĒŃĢ»ØÉś²ś¹ż³ĢÖŠµÄÖŲŅŖŌĮĻ£¬Ķ¬Ź±Ņ²æÉ×÷ĪŖ°×¹ā¼ĮŗĶÕÕĆ÷¼Į£®ĪŖĮĖĢ½¾æĆ¾ĀĮŗĻ½šÖŠø÷³É·ÖµÄŗ¬Į棬æµæµĶ¬Ń§½«Ņ»¶ØÖŹĮæµÄĆ¾ĀĮŗĻ½šĶ¶Čėµ½850mL”¢Ņ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖįÖŠ£¬ŗĻ½šČ«²æČܽā£¬Č»ŗóÓÖµĪ¼Ó5mol/LµÄNaOHČÜŅŗ£®ČōŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķÖŹĮæ£Øm£©Ėę¼ÓČėNaOHČÜŅŗµÄĢå»ż£ØV£©µÄ±ä»ÆČēĶ¼ĖłŹ¾£®| n |
| V |
| 0.15mol”Į3+0.1mol”Į2 |
| 2 |
| 0.425mol |
| 0.85L |


 ĄųŌÅŹéŅµŹī¼ŁĻĪ½ÓÄž²Ø³ö°ęÉēĻµĮŠ“š°ø
ĄųŌÅŹéŅµŹī¼ŁĻĪ½ÓÄž²Ø³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŌŖĖŲ | ½į¹¹”¢ŠŌÖŹµČŠÅĻ¢ |
| X | ¹¹³ÉÓŠ»śĪļµÄÖ÷½Ē£¬øĆŌŖĖŲµÄŅ»ÖÖŃõ»ÆĪļŗĶĘųĢ¬Ēā»ÆĪļ¶¼ŹĒµäŠĶµÄĪĀŹŅĘųĢ壮 |
| Y | ¶ĢÖÜĘŚÖŠ£Ø³żĻ”ÓŠĘųĢåĶā£©Ō×Ó°ė¾¶×ī“óµÄŌŖĖŲ£¬øƵ„ÖŹŅŗĢ¬Ź±æÉÓĆ×÷ŗĖ·“Ó¦¶ŃµÄ“«ČČ½éÖŹ£® |
| Z | ÓėYĶ¬ÖÜĘŚ£¬Ęä×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļ³ŹĮ½ŠŌ£® |
| M | ŗ£Ė®ÖŠ³żĒā”¢ŃõŌŖĖŲĶāŗ¬Įæ×ī¶ąµÄŌŖĖŲ£¬Ę䵄֏»ņ»ÆŗĻĪļŅ²ŹĒ×ŌĄ“Ė®Éś²ś¹ż³ĢÖŠ³£ÓƵÄĻū¶¾É±¾ś¼Į£® |
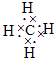
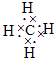
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ć¾ĀĮŗĻ½šŹĒŃĢ»ØÉś²ś¹ż³ĢÖŠµÄÖŲŅŖŌĮĻ£¬Ķ¬Ź±Ņ²æÉ×÷ĪŖ°×¹ā¼ĮŗĶÕÕĆ÷¼Į”£ĪŖĮĖĢ½¾æĆ¾ĀĮŗĻ½šÖŠø÷³É·ÖµÄŗ¬Į棬æµæµĶ¬Ń§½«Ņ»¶ØÖŹĮæµÄĆ¾ĀĮŗĻ½šĶ¶Čėµ½850 mL Ņ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖįÖŠ£¬ŗĻ½šČ«²æČܽā£¬Č»ŗóÓÖµĪ¼Ó5 mol”¤L-1µÄNaOHČÜŅŗ”£ČōŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķÖŹĮæ£Øm£©Ėę¼ÓČėNaOHČÜŅŗµÄĢå»ż£ØV£©µÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£ŗ
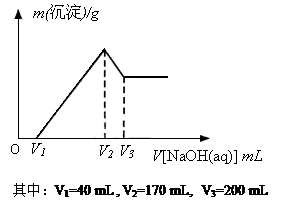
£Ø1£©ĒėŠ“³ö¼ÓČėNaOHČÜŅŗµÄĢå»żÓÉV2”śV3Ź±£¬Ėł·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ___________________ _ _______£¬²¢ÓÉ“Ė¼ĘĖćŗĻ½šÖŠĀĮµÄÖŹĮ攣
£Ø2£©¼ĘĖćŗĻ½šÖŠĆ¾µÄÖŹĮ攣
£Ø3£©ŗĻ½šŌŚČÜÓŚĻ”ĮņĖįŹ±£¬ŹĶ·Å³öµÄĘųĢåĢå»ż£Ø±ź×¼×“æö£©ŹĒ¶ąÉŁ£æ
£Ø4£©æµæµĶ¬Ń§ĖłÓĆĻ”ĮņĖįµÄĪļÖŹµÄĮæÅØ¶ČŹĒ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
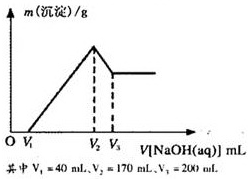 Ć¾ĀĮŗĻ½šŹĒŃĢ»ØÉś²ś¹ż³ĢÖŠµÄÖŲŅŖŌĮĻ£¬Ķ¬Ź±Ņ²æÉ×÷ĪŖ°×¹ā¼ĮŗĶÕÕĆ÷¼Į£®ĪŖĮĖĢ½¾æĆ¾ĀĮŗĻ½šÖŠø÷³É·ÖµÄŗ¬Į棬æµæµĶ¬Ń§½«Ņ»¶ØÖŹĮæµÄĆ¾ĀĮŗĻ½šĶ¶Čėµ½850mL”¢Ņ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖįÖŠ£¬ŗĻ½šČ«²æČܽā£¬Č»ŗóÓÖµĪ¼Ó5mol/LµÄNaOHČÜŅŗ£®ČōŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķÖŹĮæ£Øm£©Ėę¼ÓČėNaOHČÜŅŗµÄĢå»ż£ØV£©µÄ±ä»ÆČēĶ¼ĖłŹ¾£®
Ć¾ĀĮŗĻ½šŹĒŃĢ»ØÉś²ś¹ż³ĢÖŠµÄÖŲŅŖŌĮĻ£¬Ķ¬Ź±Ņ²æÉ×÷ĪŖ°×¹ā¼ĮŗĶÕÕĆ÷¼Į£®ĪŖĮĖĢ½¾æĆ¾ĀĮŗĻ½šÖŠø÷³É·ÖµÄŗ¬Į棬æµæµĶ¬Ń§½«Ņ»¶ØÖŹĮæµÄĆ¾ĀĮŗĻ½šĶ¶Čėµ½850mL”¢Ņ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖįÖŠ£¬ŗĻ½šČ«²æČܽā£¬Č»ŗóÓÖµĪ¼Ó5mol/LµÄNaOHČÜŅŗ£®ČōŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķÖŹĮæ£Øm£©Ėę¼ÓČėNaOHČÜŅŗµÄĢå»ż£ØV£©µÄ±ä»ÆČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2009-2010ѧğŗÓÄĻŹ”Ö£ÖŻŹŠøßŅ»£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com