

Ķ¼5-4
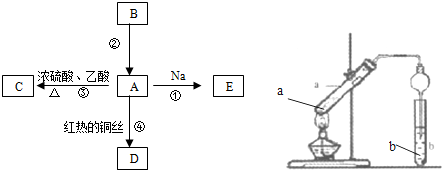
(1)ČōAĪŖ»ĘÉ«¹ĢĢ壬CÄÜŹ¹Ę·ŗģŹŌŅŗĶŹÉ«£¬DµÄĖ®ČÜŅŗÖŠ¼ÓČėHNO3Ėį»ÆµÄAgNO3ČÜŅŗÓŠ°×É«³ĮµķÉś³É”£FČÜŅŗÖŠÖ»ÓŠŅ»ÖÖČÜÖŹ£¬Ōņ£ŗ
¢Ł¹¤ŅµÉĻ·“Ó¦IŌŚ___________ÖŠ½ųŠŠ£¬·“Ó¦IµÄ»Æѧ·½³ĢŹ½ŹĒ__________________________”£
¢Ś·“Ó¦¢ņµÄĢõ¼žŹĒ_______________________________________________________”£
¢ŪD”śFµÄĄė×Ó·½³ĢŹ½ŹĒ__________________________________________________”£
(2)Čō¼×ĪŖµ»ĘÉ«¹ĢĢ壬D”¢FµÄČÜŅŗ¾ł³Ź¼īŠŌ£¬ÓĆĮ½øł²£Į§°ō·Ö±šÕŗČ”A”¢GµÄÅØČÜŅŗ²¢Ź¹ĖüĆĒ½Ó½ü£¬ÓŠ“óĮæ°×ŃĢÉś³É”£Ōņ£ŗ
¢Ł¼×µÄµē×ÓŹ½ŹĒ___________”£
¢ŚDµÄČÜŅŗÓėŅŅ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________________________________”£
¢ŪČō3.4gAÓėO2·“Ӧɜ³ÉĘųĢ¬µÄBŗĶCŹ±·Å³ö45.34kJČČĮ棬Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ_________________________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
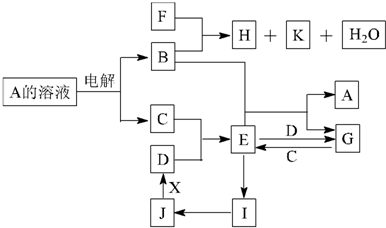
 AŹĒ»ÆѧŹµŃéŹŅÖŠ³£¼ūµÄÓŠ»śĪļ£¬ĖüŅ×ČÜÓŚĖ®²¢ÓŠĢŲŹāĻćĪ¶£»BµÄ²śĮææÉŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹µÄĖ®Ę½£®ÓŠ¹ŲĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£ŗ
AŹĒ»ÆѧŹµŃéŹŅÖŠ³£¼ūµÄÓŠ»śĪļ£¬ĖüŅ×ČÜÓŚĖ®²¢ÓŠĢŲŹāĻćĪ¶£»BµÄ²śĮææÉŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹µÄĖ®Ę½£®ÓŠ¹ŲĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£ŗ
| ||
| ¼ÓČČ |
| ||
| ¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
R”ŖX+2Na+X-R”ä![]() R-R”ä+2NaX
R-Rӊ+2NaX
R”ŖX+NaCN![]() R”ŖCN+NaX
R”ŖCN+NaX
øł¾ŻĻĀĮŠĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)AµÄ½į¹¹¼ņŹ½ŹĒ_________________£¬EµÄ½į¹¹¼ņŹ½ŹĒ_________________”£
(2)B![]() DµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________”£
DµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________”£
(3)C![]() FµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________”£
FµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ā±“śĢž·Ö×ÓÖŠµÄĀ±ĖŲŌ×ÓÓė»īĘĆ½šŹōŃōĄė×Ó½įŗĻ£¬·¢ÉśĻĀĮŠ·“Ó¦£ŗ
R”ŖX+2Na+X-R”ä![]() R-R”ä+2NaX
R-Rӊ+2NaX
R”ŖX+NaCN![]() R”ŖCN+NaX
R”ŖCN+NaX
øł¾ŻĻĀĮŠĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾»Ų“šĻĀĮŠĪŹĢā£ŗ
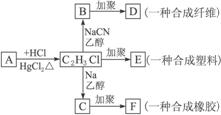
(1)AµÄ½į¹¹¼ņŹ½ŹĒ_________________£¬EµÄ½į¹¹¼ņŹ½ŹĒ_________________”£
(2)B![]() DµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________________”£
DµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________________”£
(3)C![]() FµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________________”£
FµÄ»Æѧ·½³ĢŹ½ŹĒ___________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com