
| 1000�Ѧ� |
| M |
| n |
| V |
| 1000��1.84g/ml��98% |
| 98g/mol |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
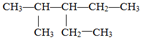 ��������
���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��50mL0.25mol/L H2SO4��50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��
��50mL0.25mol/L H2SO4��50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ� ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ��Ӧ���������� |
| B�����������ǵ���ɫ���� |
| C����ˮ��Ӧ�����Һ������ |
| D������CO2��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Һ̬HCl�����磬����HCl���ǵ���� |
| B��NH3����ˮ�γɵ���Һ�ܵ��磬����NH3�ǵ���� |
| C��SO2����ˮ�ܵ��磬����SO2�ǵ���� |
| D��BaSO4��ˮ��Һ���ѵ��磬������״̬���ܵ��磬����BaSO4�ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���ڢۢ� |
| C���٢ۢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2C2H2��g��+502��g��=4C02 ��g��+2H20 ��1����H=-2b kJ��mol-l | ||
B��C2H2��g��+
| ||
| C��2C2H2��g��+502��g��=4C02 ��g��+2H20 ��1����H=-4b kJ��mol-l | ||
| D��2C2H2��g��+502��g��=4C02 ��g��+2H20 ��1����H=b kJ��mol-l |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com