
| A�� | �����ɳ����ﵽ�����ʱ������NaOH��Һ�����Ϊ150mL | |
| B�� | ������ȫ���ܽ�ʱ�ռ���NO��������Ϊ4.48L����״���£� | |
| C�� | �μӷ�Ӧ�Ľ�����������һ����9.9g | |
| D�� | ������ȫ���ܽ�ʱ���μӷ�Ӧ����������ʵ���һ����0.6mol |
���� ��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO����������Ӧ��3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O��
��Ӧ�����Һ�м��������3mol/L NaOH��Һ��������ȫ��������Ӧ��Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������7.65g����������þ��������ͭ����������������Ϊ7.65g�������������ʵ���Ϊ$\frac{7.65g}{17g/mol}$=0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol��
A����Ӧ���������ǡ�÷�Ӧ��Ҳ������ʣ�࣬���ܼ������ɳ����ﵽ�����ʱ����NaOH��Һ�������
B�����ݵ���ת���غ����NO���ʵ������ٸ���V=nVm����NO�������
C��þ��ͭ���ܵ����ʵ���Ϊ0.225mol��������������������ĺ����йأ�
D�����ݵ�Ԫ���غ�n��HNO3��=2n������ͭ+����þ��+n��NO����
��� �⣺��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO����������Ӧ��3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O��
��Ӧ�����Һ�м��������6mol/L NaOH��Һ��������ȫ��������Ӧ��Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������7.65g����������þ��������ͭ����������������Ϊ7.65g�������������ʵ���Ϊ$\frac{7.65g}{17g/mol}$=0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol��
A������û��ʣ��ʱ�������ɵij����ﵽ�����ʱ����Һ������ΪNaNO3��������غ��֪n��NaNO3��=2n������ͭ+����þ��=0.225mol��2=0.45mol�����������غ���n��NaOH��=n��NaNO3��=0.45mol���ʴ�ʱ����������Һ�����Ϊ$\frac{0.45mol}{3mol/L}$=0.15L=150mL����������ʣ�࣬���ĵ�����������Һ�������150mL����A����
B�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ$\frac{0.225mol��2}{3}$=0.15mol����״���£�����NO�����Ϊ0.15mol��22.4L/mol=3.36L����B����
C��þ��ͭ���ܵ����ʵ���Ϊ0.225mol���ٶ�ȫΪþ������Ϊ0.225mol��24g/mol=5.4g����ȫΪͭ������Ϊ0.225mol��64g/mol=14.4g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ5.4g��m��14.4g����C����
D�����ݵ�Ԫ���غ�n��HNO3��=2n������ͭ+����þ��+n��NO��=0.225mol��2+0.15mol=0.6mol����D��ȷ��
��ѡ��D��
���� ������Ҫ���������йؼ��㣬�ۺϿ���ѧ���ĵ�ʧ�����غ㡢�����غ���ۺ����úͽ�������������������һ�����������ĺ��⣬Aѡ��Ϊ�״��㣬ѧ�����װ�����ǡ����ȫ��Ӧ���㣬�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
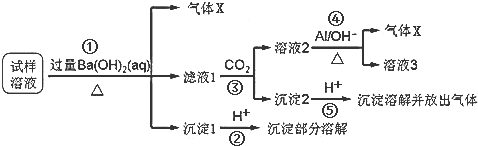
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Na2O2��SO2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2SO2�T2Na2SO4��
��Na2O2��SO2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2SO2�T2Na2SO4���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
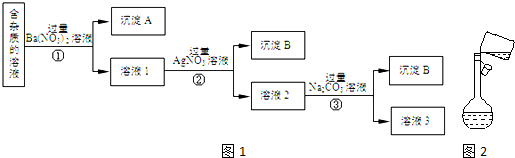
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
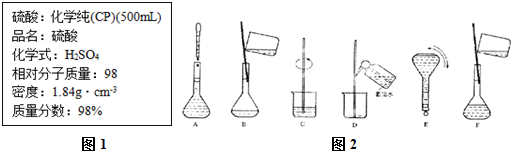
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�22g CO2����2NA����ԭ�� | |
| B�� | ��״���£�22.4L��CCl4�к��еķ�����ΪNA | |
| C�� | 1mol/L��̼������Һ�к�Na+������Ϊ2NA | |
| D�� | �����£�����0.1NA��HCl���ӵ��Ȼ�����������ˮ���100mL��Һ��������Һ��C��H+��Ϊ1mol/L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com