
 H��C��N��O��Al��S�dz���������Ԫ�أ�
H��C��N��O��Al��S�dz���������Ԫ�أ�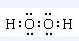 ��H��C??C��H��
��H��C??C��H������ ��1������ͬһ�����2����������ѡ�����෴�жϣ�
��2�����ۼ�Ϊԭ��֮���Թ��õ��ӶԳɼ���̼�ķǽ����Խ��������뾶��С����Ӧ������ʧȥ��õ����ӣ�
��3��ͬ��Ԫ�ص�ԭ��֮���γɷǼ��Լ���
��4������Һ��Al3+��NH4+��ˮ��ʹ��Һ�����ԣ���Al3+�� NH4+ˮ��̶ȸ���
��N������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3•H2O��
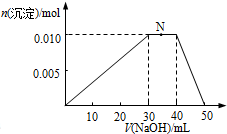
�۸���n=cV����n��Al3+ ����n��NH4+����n��SO42-����n��Ba2+����n��OH-��������SO42-��Ba2+�в����������ӵ����ʵ�����������BaSO4�����ʵ��������η�����Al3++3OH-=Al��OH��3����NH4++OH-=NH3•H2O��Al��OH��3+OH-=AlO2-+2H2O�����ݷ���ʽ��������Al��OH��3�����ʵ������������������ɹ��������ʵ�����
��� �⣺��1��Cԭ�Ӻ�������Ų�Ϊ1s22s22p2�����ʽΪ��ͼ��ʾ�� �����ڻ�̬14Cԭ���У��������2�������෴�ĵ��ӣ�
�����ڻ�̬14Cԭ���У��������2�������෴�ĵ��ӣ�
�ʴ�Ϊ��2��
��2�����ۼ�Ϊԭ��֮���Թ��õ��ӶԳɼ���̼ԭ�Ӻ�����4�����ӣ���Ԫ�صķǽ����Խ��������뾶��С����Ӧ������ʧȥ��õ����ӣ�
�ʴ�Ϊ��C��4���۵����Ұ뾶��С������ͨ���û�ʧ���Ӵﵽ�ȶ��ṹ��
��3������Ԫ�ؿ���ɶ�����ԭ�ӵĹ��ۻ�������к��Ǽ��Լ���������H2O2��C2H2������ʽ�ֱ�Ϊ ��H��C??C��H��
��H��C??C��H��
�ʴ�Ϊ�� ��H��C??C��H��
��H��C??C��H��
��4����NH4Al��SO4��2����Һ��Al3+��NH4+��ˮ��ʹ��Һ�����ԣ����ڼ��ԣ���ˮ��Al��OH��3������Al3+�� NH4+ˮ��̶ȸ���c��NH4+����c��Al3+����������Ũ���ɴ�С��˳���ǣ�c��SO42-����c��NH4+����c��Al3+����c��H+����c��OH-����
�ʴ�Ϊ��c��SO42-����c��NH4+����c��Al3+����c��H+����c��OH-�������ڼ��ԣ���ˮ��Al��OH��3����Al3+��ˮ��̶ȴ���NH4+��ˮ��̶ȣ�c��NH4+����c��Al3+����
��N������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3•H2O�����ӷ���ʽΪ��NH4++OH-=NH3•H2O��
�ʴ�Ϊ��NH4++OH-=NH3•H2O��
��10mL 1mol•L-1 NH4Al��SO4��2��Һ��Al3+ ���ʵ���Ϊ0.01mol��NH4+�����ʵ���Ϊ0.01mol��SO42-�����ʵ���Ϊ0.02mol��20mL 1.2 mol•L-1Ba��OH��2��Һ��Ba2+���ʵ���Ϊ0.024mol��OH-Ϊ0.048mol��
��SO42-+Ba2+=BaSO4������֪SO42-���㣬�ʿ��Եõ�0.02mol BaSO4��
Al3++3OH-=Al��OH��3��
0.01mol 0.03mol 0.01mol
��Ӧʣ��OH-Ϊ0.048mol-0.03mol=0.018mol��
NH4++OH-=NH3•H2O
0.01mol 0.01mol
��Ӧʣ��OH-Ϊ0.018mol-0.01mol=0.008mol��
Al��OH��3+OH-=AlO2-+2H2O
0.008mol 0.008mol
�ʵõ�Al��OH��3����Ϊ0.01mol-0.008mol=0.002mol
�����յõ�����Ϊ0.02mol+0.002mol=0.022mol��
�ʴ�Ϊ��0.022��
���� ���⿼���������Ų����ɡ���ѧ�����ͼ�����ʽ����д������Ũ�ȴ�С�Ƚϡ���ѧͼ��ѧ���㣬�Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܶȱȿ���С | B�� | ����ɫ��ζ������ | ||
| C�� | KOH��Һ������̿������������ | D�� | ��ʹ�������ɫ������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
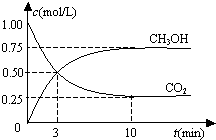
| A�� | �ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����Ϊ0.075 mol/��L•min�� | |
| B�� | �ӷ�Ӧ��ʼ��ƽ�⣬������ת����Ϊ75% | |
| C�� | ���ܱ����������Ϊ1L | |
| D�� | ���¶��£���Ӧ��ƽ�ⳣ����ֵΪ$\frac{16}{3}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | SiO2 | C | Na2O | K2O | Al2O3 | Fe2O3 |
| �������� | 59.20 | 38.80 | 0.25 | 0.50 | 0.64 | 0.16 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
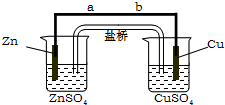 ijѧ����������ʵ��װ��̽������ʽԭ��صĹ���ԭ��������ʵ�鲽�����λش��������⣺
ijѧ����������ʵ��װ��̽������ʽԭ��صĹ���ԭ��������ʵ�鲽�����λش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
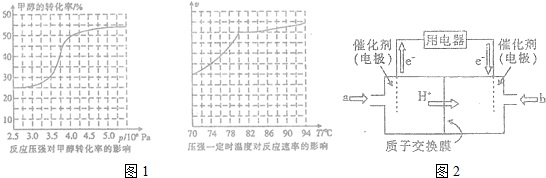
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Һȥ���������������Ĥ��Al2O3+2OH-�T2AlO2-+H2O | |
| B�� | ͭƬ����ϡ���������ɫ���壺Cu+4H++2NO3-�TCu2++2NO2��+2H2O | |
| C�� | �����������ڿ����б��ʣ�2Fe��OH��2+O2+2H2O�T2Fe��OH��3 | |
| D�� | ̼������Һ�ʼ��ԣ�CO32-+2H2O?H2CO3+2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
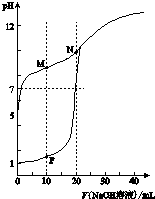 ��0.1mol•L-1 NaOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol•L-1 HCl��Һ��HX��Һ����Һ��pH�����NaOH��Һ����仯��ͼ��
��0.1mol•L-1 NaOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol•L-1 HCl��Һ��HX��Һ����Һ��pH�����NaOH��Һ����仯��ͼ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com