
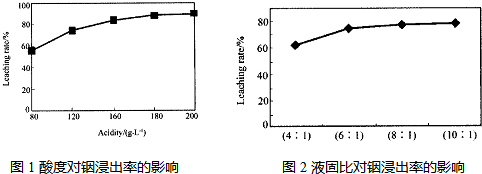
øł¾ŻĪļÖŹŠŌÖŹµÄ²īŅģ£¬ĪŅĆĒæÉŅŌæ¼ĀĒÓĆĮ½ÖÖ·½·Ø½ųŠŠĪļÖŹµÄ·ÖĄė£ŗĪļĄķ·½·ØŗĶ»Æѧ·½·Ø£®ĪļĄķ·½·ØŹĒøł¾ŻĪļÖŹĪļĄķŠŌÖŹµÄ²»Ķ¬¶ų²ÉÓƵķ֥ė·½·Ø£®Čē¹żĀĖ”¢½į¾§”¢ÕōĮó”¢ŻĶČ”µČ£®»Æѧ·½·ØŹĒĄūÓĆĪļÖŹ¼ä»ÆѧŠŌÖŹµÄ²īŅģ£¬Ń”ÓĆŗĻŹŹµÄŹŌ¼Į½ųŠŠ»Æѧ·“Ó¦£¬Č»ŗóŌŁÓĆĪļĄķ·½·Ø½«Ęä·ÖĄė£®»ÆѧŹŌ¼ĮµÄŃ”ŌńŌŌņŅ»°ć×ńŃ£ŗ¢Ł
________£»¢Ś________£»¢Ū________£®·“Ó¦½įŹųŗóÓ¦½«±»Ģį“æµÄĪļÖŹ»Öø“Ōד£® ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø
ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø »ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
»ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| ||

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖ“śĮ¶ŠæµÄ·½·ØæÉ·ÖĪŖ»š·ØŗĶŹŖ·ØĮ½“óĄą£¬ļÓŹĒÉś²śĮņĖįŠæµÄø±²śĘ·£¬ŹōÓŚø߶¾ŠŌ½šŹō£¬ŹŌ»Ų“šĻĀĮŠĻą¹ŲĪŹĢā£®
¢Å»š·ØĮ¶ŠæŹĒ½«ÉĮŠææó£ØÖ÷ŅŖŗ¬ZnS£©Ķعżø”Ń””¢±ŗÉÕŹ¹Ėü×Ŗ»ÆĪŖŃõ»ÆŠæ£¬ŌŁ°ŃŃõ»ÆŠæŗĶ½¹Ģæ»ģŗĻ£¬ŌŚ¹Ä·ēĀÆÖŠ¼ÓČČÖĮ1373-1573K£¬Ź¹ŠæÕōĮó³öĄ“”£Ö÷ŅŖ·“Ó¦ĪŖ£ŗ
2ZnS +3O2![]() 2ZnO+2SO2£» ¹Ä·ēĀÆÖŠ£ŗ2C +O2
2ZnO+2SO2£» ¹Ä·ēĀÆÖŠ£ŗ2C +O2![]() 2CO ZnO+CO
2CO ZnO+CO![]() Zn + CO2
Zn + CO2
“Ó»š·ØĮ¶ŠæÕōĮóŗóµÄ²ŠŌüÖŠŗ¬¶ąÖÖ½šŹōµ„ÖŹ¼°In2O3£¬æÉÓĆĮņĖįĢįČ”ī÷£¬Ä³ŃŠ¾æ»ś¹¹¶Ō“ĖŃŠ¾æŹż¾ŻČēĻĀ”£ŹµŃéÖŠÉę¼°µÄĖį¶Č£ØĆæÉżČÜŅŗÖŠŗ¬ĮņĖįµÄÖŹĮ棩Óėī÷µÄ½ž³öĀŹČēĻĀĶ¼1£»ĮņĖįČÜŅŗµÄĢå»żÓė¹ĢĢåµÄĢå»ż±ČČēĶ¼2
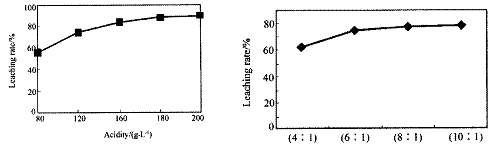
Ķ¼1Ėį¶Č¶Ōī÷½ž³öĀŹµÄÓ°Ļģ Ķ¼2Ņŗ¹Ģ±Č¶Ōī÷½ž³öĀŹµÄÓ°Ļģ
¢Łµ±Ėį¶ČĪŖ196Ź±£¬ĘäĪļÖŹµÄĮæÅضČĪŖ ”£
¢Ś“Ó½ŚŌ¼ŌĮĻŗĶ½ž³öĀŹæ¼ĀĒ£¬ŹŹŅĖµÄĖį¶ČŗĶŅŗ¹Ģ±Č·Ö±šĪŖ£ŗ_______”¢_______”£
¢ĘŹŖ·ØĮ¶ŠæµÄÖ÷ŅŖ¹¤ŅÕĮ÷³ĢĪŖ£ŗ

¢Ł“Ó±£»¤»·¾³ŗĶ³ä·ÖĄūÓĆŌĮĻ½Ē¶Č£¬ČēŗĪ“¦Ąķ»ņĄūÓĆŃĢĘų ”£
¢Ś³żČ„Ėį½ž³öŅŗÖŠµÄĢś£¬æÉÓĆH2O2Ńõ»Æ£¬ŌŁµ÷½ŚpHŹ¹Ö®ŠĪ³ÉFe(OH)3³Įµķ£¬Š“³öH2O2Ńõ»ÆFe2+µÄĄė×Ó·½³ĢŹ½ ”£
¢ŪĖį½ž³öŅŗ»¹ŗ¬ÓŠCd2+£¬ĪŖĮĖ·ĄÖ¹ļÓĪŪČ¾²¢»ŲŹÕļÓ£¬øł¾ŻĖüĆĒŠŌÖŹµÄ²īŅģ£¬æÉÓĆĒāŃõ»ÆÄĘČÜŅŗ·ÖĄė£¬ŅŃÖŖZn(OH)2ŗĶĒāŃõ»ÆĀĮŅ»ŃłŅ²¾ßÓŠĮ½ŠŌ£¬ŹŌŠ“³ö·ÖĄėµÄĄė×Ó·½³ĢŹ½____________________”¢_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖ“śĮ¶ŠæµÄ·½·ØæÉ·ÖĪŖ»š·ØŗĶŹŖ·ØĮ½“óĄą£¬ļÓŹĒÉś²śĮņĖįŠæµÄø±²śĘ·£¬ŹōÓŚø߶¾ŠŌ½šŹō£¬ŹŌ»Ų“šĻĀĮŠĻą¹ŲĪŹĢā£®
¢Å»š·ØĮ¶ŠæŹĒ½«ÉĮŠææó£ØÖ÷ŅŖŗ¬ZnS£©Ķعżø”Ń””¢±ŗÉÕŹ¹Ėü×Ŗ»ÆĪŖŃõ»ÆŠæ£¬ŌŁ°ŃŃõ»ÆŠæŗĶ½¹Ģæ»ģŗĻ£¬ŌŚ¹Ä·ēĀÆÖŠ¼ÓČČÖĮ1373-1573K£¬Ź¹ŠæÕōĮó³öĄ“”£Ö÷ŅŖ·“Ó¦ĪŖ£ŗ
2ZnS +3O2![]() 2ZnO+2SO2£» ¹Ä·ēĀÆÖŠ£ŗ2C +O2
2ZnO+2SO2£» ¹Ä·ēĀÆÖŠ£ŗ2C +O2![]() 2CO ZnO+CO
2CO ZnO+CO![]() Zn + CO2
Zn + CO2
“Ó»š·ØĮ¶ŠæÕōĮóŗóµÄ²ŠŌüÖŠŗ¬¶ąÖÖ½šŹōµ„ÖŹ¼°In2O3£¬æÉÓĆĮņĖįĢįČ”ī÷£¬Ä³ŃŠ¾æ»ś¹¹¶Ō“ĖŃŠ¾æŹż¾ŻČēĻĀ”£ŹµŃéÖŠÉę¼°µÄĖį¶Č£ØĆæÉżČÜŅŗÖŠŗ¬ĮņĖįµÄÖŹĮ棩Óėī÷µÄ½ž³öĀŹČēĻĀĶ¼1£»ĮņĖįČÜŅŗµÄĢå»żÓė¹ĢĢåµÄĢå»ż±ČČēĶ¼2
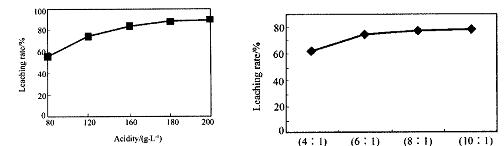
¢Łµ±Ėį¶ČĪŖ196Ź±£¬ĘäĪļÖŹµÄĮæÅضČĪŖ ”£
¢Ś“Ó½ŚŌ¼ŌĮĻŗĶ½ž³öĀŹæ¼ĀĒ£¬ŹŹŅĖµÄĖį¶ČŗĶŅŗ¹Ģ±Č·Ö±šĪŖ£ŗ_______”¢_______”£
¢ĘŹŖ·ØĮ¶ŠæµÄÖ÷ŅŖ¹¤ŅÕĮ÷³ĢĪŖ£ŗ
¢Ł“Ó±£»¤»·¾³ŗĶ³ä·ÖĄūÓĆŌĮĻ½Ē¶Č£¬ČēŗĪ“¦Ąķ»ņĄūÓĆŃĢĘų ”£
¢Ś³żČ„Ėį½ž³öŅŗÖŠµÄĢś£¬æÉÓĆH2O2Ńõ»Æ£¬ŌŁµ÷½ŚpHŹ¹Ö®ŠĪ³ÉFe(OH)3³Įµķ£¬Š“³öH2O2Ńõ»ÆFe2+µÄĄė×Ó·½³ĢŹ½ ”£
¢ŪĖį½ž³öŅŗ»¹ŗ¬ÓŠCd2+£¬ĪŖĮĖ·ĄÖ¹ļÓĪŪČ¾²¢»ŲŹÕļÓ£¬øł¾ŻĖüĆĒŠŌÖŹµÄ²īŅģ£¬æÉÓĆĒāŃõ»ÆÄĘČÜŅŗ·ÖĄė£¬ŅŃÖŖZn(OH)2ŗĶĒāŃõ»ÆĀĮŅ»ŃłŅ²¾ßÓŠĮ½ŠŌ£¬ŹŌŠ“³ö·ÖĄėµÄĄė×Ó·½³ĢŹ½____________________”¢_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø8·Ö£©ĻÖ“śĮ¶ŠæµÄ·½·ØæÉ·ÖĪŖ»š·ØŗĶŹŖ·ØĮ½“óĄą£¬ļÓŹĒÉś²śĮņĖįŠæµÄø±²śĘ·£¬ŹōÓŚø߶¾ŠŌ½šŹō£¬ŹŌ»Ų“šĻĀĮŠĻą¹ŲĪŹĢā.
£Ø1£©»š·ØĮ¶ŠæŹĒ½«ÉĮŠææó£ØÖ÷ŅŖŗ¬ZnS£©Ķعżø”Ń””¢±ŗÉÕŹ¹Ėü×Ŗ»ÆĪŖŃõ»ÆŠæ£¬ŌŁ°ŃŃõ»ÆŠæŗĶ½¹Ģæ»ģŗĻ£¬ŌŚ¹Ä·ēĀÆÖŠ¼ÓČČÖĮ1373-1573K£¬Ź¹ŠæÕōĮó³öĄ“”£
Ö÷ŅŖ·“Ó¦ĪŖ£ŗ2ZnS +3O22ZnO+2SO2 £»¹Ä·ēĀÆÖŠ£ŗ2C+O2
2CO
¹Ä·ēĀÆÖŠ£ŗZnO+COZn + CO2
“Ó»š·ØĮ¶ŠæÕōĮóŗóµÄ²ŠŌüÖŠŗ¬¶ąÖÖ½šŹōµ„ÖŹ¼°In2O3£¬æÉÓĆĮņĖįĢįČ”ī÷£¬Ä³ŃŠ¾æ»ś¹¹¶Ō“ĖŃŠ¾æŹż¾ŻČēĻĀ”£ŹµŃéÖŠÉę¼°µÄĖį¶Č£ØĆæÉżČÜŅŗÖŠŗ¬ĮņĖįµÄÖŹĮ棩Óėī÷µÄ½ž³öĀŹČēĻĀĶ¼1£»ĮņĖįČÜŅŗµÄĢå»żÓė¹ĢĢåµÄĢå»ż±ČČēĶ¼2
¢Łµ±Ėį¶ČĪŖ196Ź±£¬ĘäĪļÖŹµÄĮæÅضČĪŖ ”£
¢Ś“Ó½ŚŌ¼ŌĮĻŗĶ½ž³öĀŹæ¼ĀĒ£¬ŹŹŅĖµÄĖį¶ČŗĶŅŗ¹Ģ±Č·Ö±šĪŖ£ŗ ”¢ ”£
£Ø2£©ŹŖ·ØĮ¶ŠæµÄÖ÷ŅŖ¹¤ŅÕĮ÷³ĢĪŖ
¢ŁĮņĖį½žČ”µÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
¢Ś“Ó±£»¤»·¾³ŗĶ³ä·ÖĄūÓĆŌĮĻ½Ē¶Č£¬ČēŗĪ“¦Ąķ»ņĄūÓĆŃĢĘų ”£
¢Ū³żČ„Ėį½ž³öŅŗÖŠµÄĢś£¬æÉÓĆH2O2Ńõ»Æ£¬ŌŁµ÷½ŚpHŹ¹Ö®ŠĪ³ÉFe(OH)3³Įµķ£¬Š“³öH2O2Ńõ»ÆFe2+µÄĄė×Ó·½³ĢŹ½ ”£
¢ÜĖį½ž³öŅŗ»¹ŗ¬ÓŠCd2+,ĪŖĮĖ·ĄÖ¹ļÓĪŪČ¾²¢»ŲŹÕļÓ£¬øł¾ŻĖüĆĒŠŌÖŹµÄ²īŅģ£¬æÉÓĆĒāŃõ»ÆÄĘČÜŅŗ·ÖĄė£¬ŅŃÖŖZn(OH)2ŗĶĒāŃõ»ÆĀĮŅ»ŃłŅ²¾ßÓŠĮ½ŠŌ£¬ŹŌŠ“³ö·ÖĄėµÄĄė×Ó·½³ĢŹ½ ___________ ”¢ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com