
1)ͼ��¬���ɶ��ɺ�����γɵĵ��������ڴ�����CO2�����ľ���������ɺ����(��Ҫ�ɷ���̼���)����ˮ��ʴ����ԭ����(�û�ѧ����ʽ��ʾ)____________________________��
(2)����ЧӦ���º�ƽ����������������ЧӦ����Ϊԭ����Ҫ�ǣ�______________________________________________��
(3)Ϊ�˿�������ЧӦ��������ѧ������˲��ٷ��������롣���˸���Һ̬CO2�ܶȴ��ں�ˮ�ܶȵ���ʵ�����뽫CO2Һ������������ף��Լ�С������CO2��Ũ�ȡ�ΪʹCO2Һ�����ɲ��õĴ�ʩ��__________��
A����ѹ�����¡��������� B����ѹ������
C����ѹ������ D����ѹ������
(4)��ѧ�������ڶ�����̼�ġ����ת���������о����ѹ���Ķ�����̼ת��Ϊ��������������ʡ��罫CO2��H2��14�ı�����ϣ�ͨ�뷴Ӧ�������ʵ��������·�Ӧ���ɻ��һ����Ҫ����Դ����������»�ѧ����ʽ��
CO2��4H2����(����)��2H2O
(5)��Ч�ؼ���������CO2�������ӵ���̬ѧ��ʩ��__________��
A��ʹ����Ȼ����ȼ��
B������ȫ���˿�����
C��ֲ�����֣�����ɭ��
D����������ú��ʯ�͵�ȼ��
������(1)���ڿ�����CO2����������ʹ��ˮ���ܽ��CO2���࣬������ǿ��ʹɺ�����ܵ����ظ�ʴ��
(2)����ȼ��ú��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ��ǿ�����CO2����������������ЧӦ����Ҫԭ��
(3)���¡���ѹ��ʹCO2Һ����
(4)��ԭ���غ��֪��ȱ������ΪCH4��
(5)��Ȼ��������CO2����Ҫ������ֲ��Ĺ�����ã�����ֲ�����֣�����ɭ�ֿ���Ч�ؼ���������CO2�Ĵ������ӡ�
�𰸡�(1)CaCO3��CO2��H2O===Ca(HCO3)2
(2)����ȼ��ú��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ�ʹ������CO2�ĺ�������
(3)D
(4)CH4
(5)C


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ�У���ȷ���� �� ��
A����Mg��HCO3��2��Һ�м��������NaOH��Һ
Mg2++2HCO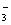 +2OH—
+2OH— MgCO3��+ CO32��+2H2O
B���廯������Һ��ͨ��������������2Fe2++Cl2=2Fe3++2Cl-
C��������CO2ͨ��ϡ��̼������Һ�У�CO2+CO32��+H2O 2HCO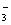
D����NH4Al��SO4��2��Һ�е���Ba��OH��2ʹSO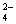 ��Ӧ��ȫ
��Ӧ��ȫ
2Ba2++4OH—+Al3++2SO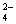
2BaSO4��+AlO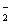 +2H2O
+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
a��b��c����Ԫ�أ�a��bͬ���ڣ�a��cͬ���壬����ԭ�ӵ�����������֮��Ϊ16����������Ԫ�ؿ�����(����)
A��P��S��N B��Cl��S��F
C��S��Cl��O D��K��Ca��Na
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ҹ�������н�ֹ����ʹ�ú�Ǧ���ͣ�����Ҫԭ����(����)
A���������ȼ��Ч�� B���������ͳɱ�
C������Ǧ��Ⱦ���� D��Ǧ��Դ��ȱ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Խ��ܼ���Ϊ�����ĵ�̼�����DZ������ɳ�����չ��ս�Ծٴ롣��������Υ����չ��̼���õ���(����)
A����չ���ܺ�̫����
B������������Ʒ��ʹ��
C�����ԭ�������ʣ���չ��ɫ��ѧ
D�������ô�Һ̬�л������ˮ���ܼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ����(����)
A����������6��̼̼����ȫ��ͬ
B������һ������������Cl2����ȡ����Ӧ
C������ŨHNO3��ŨH2SO4�Ļ������50��60��C�ܷ����ӳɷ�Ӧ
D�������������к������ֻ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����̼����������Ϊ92.3%�����������ܶ�����ͬ������H2�ܶȵ�39����
(1)�����ķ���ʽΪ__________���ṹ��ʽΪ__________������ӹ���Ϊ________��
(2)�����ڿ�����ȼ�յ�����Ϊ________________________________________________________________________��
(3)������Ũ���ᷴӦ�Ļ�ѧ����ʽΪ(�л���д�ṹ��ʽ)________________________________���䷴Ӧ����Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ճ�����ڱ���һ����Ϊ���ķ���ϩ�ĸ߷��Ӳ���Ϳ�㣬�ò�ճ�����ղ�ʱ����ճ�����ս������й��ھ��ķ���ϩ��˵����ȷ����(����)
A�����ķ���ϩ�����к���˫��
B�����ķ���ϩ�ĵ����Dz�������
C�����ķ���ϩ�з�������������76%
D�����ķ���ϩ�Ļ�ѧ���Խϴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�ϳɰ���Ӧ��Ũ���������£�
| �� N2����3H2 | |||
| ��ʼŨ��(mol/L) | 1.0 | 3.0 | 0 |
| 2 sĩŨ��(mol/L) | 0.6 | 1.8 | 0.8 |
���ð���Ũ�ȵ���������ʾ�û�ѧ��Ӧ������ʱ��������Ϊ
(����)��
A��0.2 mol/(L��s) B��0.4 mol/(L��s)
C��0.6 mol/(L��s) D��0.8 mol/(L��s)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com