
����Ŀ������ͼ��ʾװ�òⶨþ����Ʒ�е���þ���������������������ᷴӦ���������壩
���������գ�
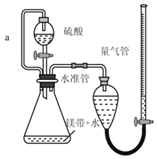
��1��������������Ŀ����_____
��2������a��������_____
��3��ȡ����þ����Ʒ�ֱ����ʵ�飬�������ݼ��±���
ʵ����� | þ��������g�� | ���������mL�����ѻ���ɱ�״���� |
1 | 0.053 | 44.60 |
2 | 0.056 | 47.05 |
����þ������������_____��������3λС����
��4������ⶨ���ƫ�ߣ����ܵ�ԭ����_____����ѡ���ţ�
a��װ��©��
b��δ��ȴ�����¼�����
c��þ���к�������þ
d��ĩ����ʱ�����ܵ�Һ�����ˮ��
���𰸡���֤þ����ȫ��Ӧ ƽ����ѹ��ʹ����˳�����£������������������������������������� 0.901 bd
��������
ʵ��ԭ��������þ�����������ʵ�����ȣ�ͨ���ų�Һ����������������������֪���������ʵ�����ͨ�����������ʵ������þ���������Ӷ������Ʒ��þ������������
��1��ʵ������Ҫͨ�����������ʵ������þ���������Ӷ������Ʒ��þ���������������Լ����������������ȷ��Mg��ȫ��Ӧ��
��2���淴Ӧ�Ľ��У���ƿ���������࣬ѹǿ����Һ©���е�����������£�Ϊ��ƽ����ѹ���õ���a����Һ©������ƿ�����������Ϳ���ʹ����˳�����¡����⣬���ڵ���������ռһ���������������a�����ⲿ����������ת�Ƶ���Һ©����Ӷ���������������������������������������Ե���a��������ƽ����ѹ��ʹ����˳�����º������������������������������������
��3��þ����ƽ������Ϊ��![]() ��0.0545g������������ƽ�����Ϊ��
��0.0545g������������ƽ�����Ϊ��![]() ��45.825mL�����ݷ�Ӧ��ϵʽMg��H2��֪��n��Mg����n��H2������Ʒ��Mg������Ϊ��24g/mol��
��45.825mL�����ݷ�Ӧ��ϵʽMg��H2��֪��n��Mg����n��H2������Ʒ��Mg������Ϊ��24g/mol��![]() ����0.04910������þ�����������ǣ�
����0.04910������þ�����������ǣ�![]() ��100%��0.901��
��100%��0.901��
��4��a��װ��©�����������������С���ⶨ���ƫ�ͣ���a����
b��δ��ȴ�����¼�������������������ԭ����֪���ⶨ���������ƫ�ⶨ���ƫ�ߣ���b��ȷ��
c��þ���к�������þ���������������ƫС���ⶨ���ƫ�ͣ���c����
d��ĩ����ʱ�����ܵ�Һ�����ˮ�ܣ����¶������ƫ�ⶨ���ƫ�ߣ���d��ȷ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ṩ���鳣����ʵ��װ��ʾ��ͼ�������й�������ȷ����
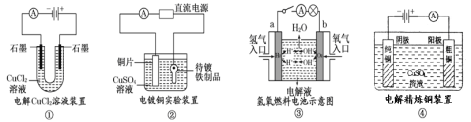
A.װ�â�������������ɫ��ζ����ð��
B.װ�â��е�ͭƬӦ��ֱ����Դ�ĸ�������
C.װ�â��У������ҺΪKOH��Һ����缫a�ķ�Ӧʽ��H2��2e��+2OH��=2H2O
D.װ�â��������ٵ�����һ�������������ӵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)���������մ�����������ɫ���ĵ�б���壬�۵�48�档���������(Na2S2O3)����Ϊ����ҵ�Ķ�Ӱ������Ӧԭ��ΪAgBr+2Na2S2O3=Na3[Ag(S2O3)2]+NaBr��
��Ϊ�˴ӷ϶�ӰҺ����ȡ AgNO3���������ʵ�����̡�
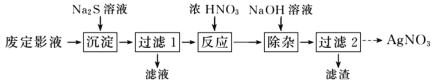
(1)������������������ Ag2S ���������������ȫ�IJ�����________��
(2)����Ӧ�������л����ɵ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ________��
(3)������ 2������Һ��ȡ AgNO3����IJ���������Ũ������ȴ�ᾧ��________��________�����
����ͼ��ʵ����ģ�ҵ�Ʊ� Na2S2O3 ��װ��ͼ��
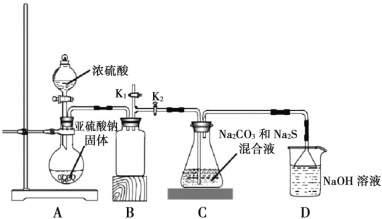
����ͼʾ�ش��������⣺
(4)װ�� A ��ʢ���������ƹ���IJ�������������________��װ�� B ��������________��
(5)��Һ©������ֱ���� 98����Ũ���ᣬ��ƿ�й����ײ�������顱����ʹ��Ӧ���ʱ�������������顱�����ԭ����________��
(6)���� K1���ܵ�Ŀ����Ϊ�˷�ֹ���װ��ʱ��ɿ�����Ⱦ���������������________��
(7)��������ƻ������ڳ�ȥ����Ƥ��ʱ�������ظ����Σ����仹ԭ�� Cr3+�������ϴ���1mol Cr2O72-��Ҫ Na2S2O3������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����������е㣺117.2�棬����ȩ�е㣺75.7�棻���������ϳ�����ȩ�ķ�Ӧ��![]() ��װ����ͼ������˵������ȷ����
��װ����ͼ������˵������ȷ����

A.Ϊ��ֹ�����һ��������Ӧ������Na2Cr2O7������Һ��μ�����������
B.��������������м������������ƣ��ɼ��������Ƿ���������
C.���¶ȼ�1ʾ��Ϊ90��95�棬�¶ȼ�2ʾ����76������ʱ�ռ�����
D.��������õĴ�����ȩ�У�������ˮCaCl2���壬���ˣ������ᴿ����ȩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��W��X��Y��Z��ԭ��������������X��ԭ�Ӱ뾶�����ж���������Ԫ�������ģ�W�ĺ����������X��Z������������֮����ȣ�Y��ԭ��������Z��������������2������W��X��Y����Ԫ���γɵĻ�����M�Ľṹ��ͼ��ʾ������������ȷ����
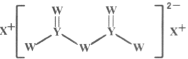
A.Ԫ�طǽ�����ǿ����˳��ΪW��Y��Z
B.Y���ʵ��۵����X����
C.������M��W��������8�����ȶ��ṹ
D.W�ļ��⻯���ȶ��Ա�Y�ļ��⻯���ȶ���ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��Ƽ������������һ�ֵ��װ�ã��ܽ�����(C3H8O3)�Ͷ�����̼ת��Ϊ����ȩ(C3H6O3)�ͺϳ�����ԭ����ͼ��ʾ������˵����ȷ����
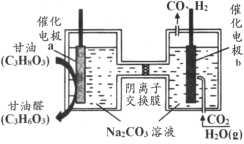
A.���缫b���Դ��������
B.���ʱ���缫a������pH����
C.���ʱ������������Ĥ��a��Ǩ��
D.���ɵĸ���ȩ��ϳ��������ʵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳɰ��ǽ��������������⡣�ش��������⣺
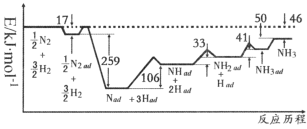
(1)��ѧ���о���������ý���ϳɰ��ķ�Ӧ������ͼ��ʾ�����������ڴ�������������á�ad����ʾ����ͼ�������խС�IJ��֣�����������![]() �ķ�ʽ��ʾ����ͼ��֪�ϳɰ���ӦN2(g)+3H2(g)
�ķ�ʽ��ʾ����ͼ��֪�ϳɰ���ӦN2(g)+3H2(g)![]() 2NH3(g)��
2NH3(g)��![]() =_______kJ��mol��1����Ӧ���������IJ���Ļ�ѧ����ʽΪ____________��
=_______kJ��mol��1����Ӧ���������IJ���Ļ�ѧ����ʽΪ____________��
(2)��ҵ�ϳɰ���ӦΪ��N2(g)+3H2(g) ![]() 2NH3(g)�������������V(N2)��V(H2)=1��3ʱ��ƽ��������NH3�����ʵ����������¶Ⱥ�ѹǿ�仯�Ĺ�ϵ��ͼ��ʾ��
2NH3(g)�������������V(N2)��V(H2)=1��3ʱ��ƽ��������NH3�����ʵ����������¶Ⱥ�ѹǿ�仯�Ĺ�ϵ��ͼ��ʾ��
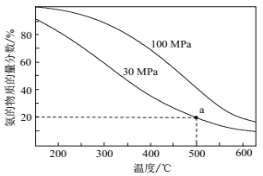
��500��ʱ��
��ƽ�ⳣ��KP(30MPa)________KP(100MPa)��(���������=����������)
��30MPaʱ��������ƽ��ת����Ϊ_________(�������3λ��Ч����)����ƽ���ѹ��ʾƽ�ⳣ��KP=_______________(�г�����ʽ���ɣ����ػ���)��
(3)��ѧ�����õ�ⷨ�ڳ��³�ѹ��ʵ�ֺϳɰ�������ʱ���������۷�Ӧ������ͼ��ʾ�����е��ҺΪ�ܽ�����������﮺��Ҵ����л���Һ��
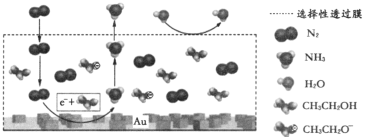
������������NH3�ĵ缫��ӦʽΪ_____________��
������˵����ȷ����_______________(����)��
A.��������﮵���������ǿ������
B.ѡ������Ĥ������N2��NH3ͨ������ֹH2O����װ��
C.���ֵ���ǿ�Ȳ��䣬������Һ���¶ȣ����Լӿ��ⷴӦ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£�������ͨ������������Һ�У���Ӧ��õ��Ȼ��أ�������أ�����صĻ����Һ�����ⶨClO��ClO3���ӵ����ʵ�����Ũ��֮��Ϊ1:2�����������������ط�Ӧʱ������ԭ����ԭ���뱻��������ԭ�ӵ����ʵ���֮��Ϊ(���� )
A. 10:3B. 11:3C. 2:3D. 4:3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��W��X��Y��Z��ԭ�Ӱ뾶��������X��Yͬ���壬��X��Yԭ������֮����W��Zԭ������֮�͵�2����m��n��p��r������ЩԪ����ɵĶ�Ԫ������ס�������������Ԫ�ض�Ӧ�ĵ��ʣ�n�����ڳ����¾��ǵ���ɫ���塣�������ʼ��ת����ϵ��ͼ��ʾ(������������ʡ��)������˵����ȷ����
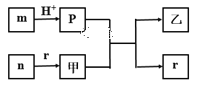
A.�����Ӱ뾶��С��X>Y>Z
B.���ȶ��ԣ�r>p
C.��YԪ�صĺ�����һ������ǿ��
D.��Ԫ������n �Ǽ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com