
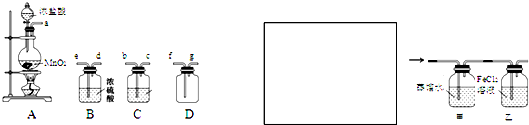
ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅ”¢±ūČżøöÉÕ±ŅĄ“Ī·Ö±šŹ¢·Å109g5.51£„µÄNaOHČÜŅŗ”¢×ćĮæµÄCuSO4ČÜŅŗŗĶ200g10.00£„µÄK2SO4ČÜŅŗ£®µē¼«¾łĪŖŹÆÄ«µē¼«”£

½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47£„£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£¾Ż“Ė»Ų“šĪŹĢā£ŗ
£Ø1£©µē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ___________________________________”£
£Ø2£©µē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×“æöĻĀµÄĢå»żĪŖ__________________£¬“ĖŹ±¼×ÉÕ±ÖŠNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ£ØÉčČÜŅŗµÄĆܶČĪŖ1g/cm3£©_______________”£
£Ø3£©µē¼«cµÄÖŹĮæ±ä»ÆŹĒ___________g£¬ÓūŹ¹µē½āŗóŅŅÖŠµÄµē½āŅŗ»Öø“µ½ĘšŹ¼×“Ģ¬£¬Ó¦øĆĻņČÜŅŗÖŠ¼ÓČėŹŹĮæµÄ___________£ØĢī×ÖÄø±ąŗÅ£©”£
A.Cu(OH)2 B.Cu2O C. CuCO3 D. Cu2(OH)2CO3
£Ø4£©ĘäĖūĢõ¼ž²»±ä£¬Čē¹ū°ŃŅŅ×°ÖĆøÄĪŖµē½ā¾«Į¶Ķ£¬Ōņcµē¼«µÄ²ÄĮĻĪŖ___________£¬dµē¼«µÄ²ÄĮĻĪŖ______”£
£Ø1£© 4OH££ 4e££½ 2H2O + O2”ü£Ø2·Ö£©
£Ø2£©5.6 L£Ø2·Ö£© 1.5mol/L£Ø2·Ö£©
(3)32£Ø2·Ö£© C£Ø1·Ö£©
(4)¾«Ķ»ņ“æĶ£Ø1·Ö£© “ÖĶ£Ø1·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻŅŅÖŠcµē¼«ÖŹĮæŌö¼ÓæÉÖŖcµē½āĪŖŅõ¼«£¬ŌņMµē¼«ĪŖµēŌ“µÄøŗ¼«£¬Nµē¼«ĪŖÕż¼«£¬bµē¼«ĪŖŃō¼«£¬·¢ÉśOH‾Ź§Č„µē×ӵķ“Ó¦£ŗ4OH££ 4e££½ 2H2O + O2”ü”£
£Ø2£©µē½āŗó200g10.00£„µÄK2SO4ČÜŅŗÅØ¶Č±äĪŖ10.47£„£¬Ōņµē½āµÄH2OµÄÖŹĮæĪŖ200g -200g”Į10.00£„”Ā10.47£„=9g£¬µē½āH2OŹ±Óė×ŖŅʵē×ӵĶŌÓ¦¹ŲĻµĪŖ£ŗH2O~2e‾£¬Ōņn(e‾)=2”Į9g”Ā18g/mol=1mol£¬øł¾Żµē¼«b·¢Éś·“Ó¦£ŗ4OH££ 4e££½ 2H2O + O2”ü£¬æÉĒó³öÉś³ÉµÄĘųĢå£ŗV(O2)=1mol”Ā4”Į22.4L/mol=5.6L£»¼×ÉÕ±ÖŠNaOHČÜŅŗŅ²µē½āĮĖ9gH2O£¬ČÜŅŗĢå»żĪŖ£ŗ£Ø109g-9g£©”Ā1000g/L=0.1L£¬c(NaOH)=109g”Į5.51%”Ā40g/mol”Ā0.1L=1.5mol•L‾1”£
£Ø3£©µē¼«cĪŖŅõ¼«£¬·¢Éś·“Ó¦£ŗCu2++2e‾=Cu£¬Éś³ÉµÄCuÖŹĮæĪŖm(Cu)=1mol”Ā2”Į64g/mol=32g£»µē½āÉś³ÉH2SO4”¢O2”¢Cu£¬øł¾Ż³öĄ“Ź²Ć“¼ÓŹ²Ć“µÄŌŌņ£¬¼ÓČėCuCO3ŗóæÉŹ¹µē½āŗóŅŅÖŠµÄµē½āŅŗ»Öø“µ½ĘšŹ¼×“Ģ¬”£
£Ø4£©µē½ā¾«Į¶Ķ£¬“æĶ×÷Ņõ¼«£¬µē¼«“ÖĶ×÷Ńō¼«£¬¹Źcµē¼«µÄ²ÄĮĻĪŖ“æĶ£¬dµē¼«µÄ²ÄĮĻĪŖ“ÖĶ”£
æ¼µć£ŗ±¾Ģāæ¼²éµē¼«·½³ĢŹ½µÄŹéŠ“”¢µē½ā²śĪļµÄ¼ĘĖć”¢µē¼«²ÄĮĻµÄŃ”Ōń”¢µē½āÖŹČÜŅŗ»Ųø“¼ÓČėĪļÖŹµÄŃ”Ōń”£


 100·Ö“³¹ŲĘŚÄ©³å“ĢĻµĮŠ“š°ø
100·Ö“³¹ŲĘŚÄ©³å“ĢĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”¼ĆÄžŹŠ×Ž³Ē¶žÖŠø߶žÉĻѧʌʌ֊֏Įæ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅĮ½øöÉÕ±·Ö±šŅĄ“ĪŹ¢·Å200mL±„ŗĶŹ³ŃĪĖ®”¢×ćĮæµÄAgNO3ČÜŅŗ£¬a”¢b”¢c”¢dĖÄøöµē¼«¾łĪŖŹÆÄ«µē¼«”£½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠdµē¼«ÖŹĮæŌö¼ÓĮĖ2.16g”£¾Ż“Ė»Ų“šĪŹĢā£ŗ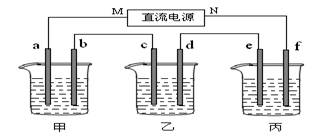
£Ø1£©µēŌ“µÄN¶ĖĪŖ ¼«£»
£Ø2£©µē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
£Ø3£©µē¼«cÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“Ģ¬ĻĀµÄĢå»ż£ŗ £»
£Ø4£©¼×ČÜŅŗµÄĒāŃõøłĄė×ÓÅضČĪŖ £ØÉčČÜŅŗĢå»żČŌĪŖ200mL)£»
£Ø5£©ÓūŌŚ±ūÉÕ±ÖŠŹµĻÖĢśµÄ±ķĆę¶ĘÉĻŅ»²ćĶ£¬Ōņµē½āÖŹČÜŅŗĪŖ £¬eµē¼«µÄ²ÄĮĻŹĒ£ŗ £¬fµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÖŲĒģŹŠĶņÖŻ¶žÖŠøßČżĒļ¼¾æŖѧ²āŹŌ»Æѧ£ØĄķ£©ŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©A”¢B”¢CČżÖÖĒæµē½āÖŹ£¬ĖüĆĒŌŚĖ®ÖŠµēĄė³öµÄĄė×ÓČēĻĀ±ķĖłŹ¾£ŗ
| ŃōĄė×Ó | Na+”¢K+”¢Cu2+ |
| ŅõĄė×Ó | SO42”Ŗ”¢OH£ |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½Ī÷Ź”°×šŲ֎֊ѧø߶žĻĀѧʌµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅ”¢±ūČżøöÉÕ±ŅĄ“Ī·Ö±šŹ¢·Å100 g 5.00%µÄNaOHČÜŅŗ”¢×ćĮæµÄCuSO4ČÜŅŗŗĶ100 g 10.00%µÄK2SO4ČÜŅŗ£¬µē¼«¾łĪŖŹÆÄ«µē¼«”£


 £Ø1£©½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£
£Ø1£©½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÅضČĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£
¾Ż“Ė»Ų“šĪŹĢā£ŗ ¢ŁµēŌ“µÄN¶ĖĪŖ ¼«£»
¢ŁµēŌ“µÄN¶ĖĪŖ ¼«£» ¢Śµē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
¢Śµē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £» ¢Ūµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ
¢Ūµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ
 ¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ g£»
¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ g£» £Ø2£©Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ
£Ø2£©Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ  ”£
”£ ¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄpHČēŗĪ±ä»Æ”££ØĢīŌö“󣬼õŠ”»ņ²»±ä£©
¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄpHČēŗĪ±ä»Æ”££ØĢīŌö“󣬼õŠ”»ņ²»±ä£© ¼×ČÜŅŗ £»
¼×ČÜŅŗ £» ŅŅČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£»
ŅŅČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£» ±ūČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£»
±ūČÜŅŗ£ß£ß£ß£ß£ß£ß£ß£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«Ź”¼ĆÄžŹŠø߶žÉĻѧʌʌ֊֏Įæ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅĮ½øöÉÕ±·Ö±šŅĄ“ĪŹ¢·Å200mL±„ŗĶŹ³ŃĪĖ®”¢×ćĮæµÄAgNO3ČÜŅŗ£¬a”¢b”¢c”¢dĖÄøöµē¼«¾łĪŖŹÆÄ«µē¼«”£½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠdµē¼«ÖŹĮæŌö¼ÓĮĖ2.16g”£¾Ż“Ė»Ų“šĪŹĢā£ŗ

£Ø1£©µēŌ“µÄN¶ĖĪŖ ¼«£»
£Ø2£©µē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
£Ø3£©µē¼«cÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“Ģ¬ĻĀµÄĢå»ż£ŗ £»
£Ø4£©¼×ČÜŅŗµÄĒāŃõøłĄė×ÓÅضČĪŖ £ØÉčČÜŅŗĢå»żČŌĪŖ200mL)£»
£Ø5£©ÓūŌŚ±ūÉÕ±ÖŠŹµĻÖĢśµÄ±ķĆę¶ĘÉĻŅ»²ćĶ£¬Ōņµē½āÖŹČÜŅŗĪŖ £¬eµē¼«µÄ²ÄĮĻŹĒ£ŗ £¬fµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com