
��.�����й�˵������ȷ����________��
A����ͬ���͵����Ӿ��壬������Խ���γɵľ���Խ�ȶ�
B��NH3��H3O+�ǵȵ����壬��˽ṹ����������
C�����ǻ�����ȩ�е���ڶ��ǻ�����ȩ��ԭ����ǰ�ߴ��ڷ�����������ߴ���
���Ӽ����
D��H3O����HF2����[Ag(NH3)2]���о�������λ��
��.̼���仯��������Ȼ���й㷺���ڡ�
(1)��̬̼ԭ�ӵļ۵����Ų�ͼ�ɱ�ʾΪ ������������������ͬ
δ�ɶԵ������Ĺ��ɽ����� ����Ԫ�ط��ţ�
(2)��һ�����ܣ�C��N��O��F����Ԫ���ɴ�С˳��___ _ ��
ԭ���� ��
HCN��NF3���ӹ��ͷֱ��� ��
(3)��������ˮ���ӵĿռ����з�ʽ����ʯ�������ơ�ÿ��������ƽ��ռ��________��ˮ���ӣ�����������ʯ�������з�ʽ��ͬ��ԭ����__________________________��
(4)C60�ľ����У�����Ϊ���������ѻ�����֪������C60�������ļ����̾���Ϊ
d cm���ɼ���C60������ܶ�Ϊ________g/cm3��
(5)��д��һ����Ӧ����ʽ�Ա������Ӧǰ̼ԭ�ӵ��ӻ���ʽΪsp2����Ӧ���Ϊsp3��________________________________��
��֪ʶ�㡿���ʽṹ������ ����ṹ E3 E4 E5
���𰸽���������.D
��.(1)  Ti Ni
Ti Ni
(2) F N O C �ǽ�����F��O��N��C���μ�������N����P�Dz������ṹ���ȶ��ԣ����һ�����ܷ�����O��
(3)8��ÿ��ˮ���������ڵ�4��ˮ�����Խ��ʯ̼ԭ�����Ƶijɼ���λ�γ����
(4)
(5)CH2===CH2��Br2����BrCH2CH2Br
��������A����ͬ���͵����Ӿ��壬������Խ���γɵľ���Խ�ȶ���ȷ��NH3��H3O+������10�����ӣ���Ϊ�ȵ����壬���ι��;�Ϊ�����Σ���B��ȷ��D�H3O+��H��O��[Ag��NH3��2]+��Ag��N������λ��HF2���в�������λ�����ʴ�ΪD��
��1��̼ԭ�Ӻ�����6�����ӣ������4�������ݹ���ԭ��������ԭ���ͺ��ع����д����̬̼ԭ�ӵļ۵����Ų�ͼ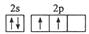 ��
��
��2��ͬ����Ԫ�ص�һ�����ܳ��������ƣ���N����P�Dz������ṹ���ȶ��ԣ����һ�����ܷ�����O�����Ե�һ�����ܴ�С˳��Ϊ��F N O C ��
��3��ÿ��������ƽ��ռ�з��Ӹ���=4+1/8��8+1/2��6=8��H2O����ԭ���к���2���Ҽ���2���µ��Ӷԣ����ʯ��ÿ��̼ԭ�Ӻ���4���Ҽ���û�йµ��Ӷԣ�����ˮ�е�O�ͽ��ʯ�е�C����sp3�ӻ�����ˮ���Ӽ��������з����ԣ�ÿ��ˮ������һ����ԭ�ӿ��Ժ�����2��ˮ�����е���ԭ���γ�2�������2����ԭ�ӿ��Ժ�����2��ˮ�����е���ԭ���γ����������ÿ��ˮ���ӿ������ڵ�4��ˮ�����γ�4����������±���������ʯ���������з�ʽ���ƣ�
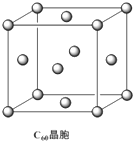
��4��������C60���Ӽ����̾���Ϊd cm�����ⳤΪ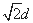
���������=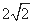 d3cm3���þ�����C60���Ӹ���=8��1/8+6��1/2=4��
d3cm3���þ�����C60���Ӹ���=8��1/8+6��1/2=4��
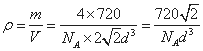
��5��sp2�ӻ���̼Ϊ�γ�˫��̼ԭ�ӣ�sp3�ӻ���̼Ϊ�γɵ���̼ԭ�ӣ���������ϩ��Br2��H2��HX��H2O�ȵļӳɷ�Ӧ��CH2═CH2+Br2-��BrCH2CH2Br��
��˼·�㲦�����⿼�����ʽṹ�����ʣ����鿼����ԭ�ӡ����ӡ�����ṹ�����ʵ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�����ȫȼ�գ�����CO2��H2O����12 g���л�����ȫȼ�պ�IJ���ͨ��ŨH2SO4��ŨH2SO4����14.4 g����ͨ����ʯ�ң���ʯ������26.4 g�����л������ʽΪ(����)
A��C4H10 B��C2H6O
C��C3H8O D��C2H4O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ϢϢ���,����˵������ȷ����
A�������Cl2����ˮ����ʹ�ʻ���ɫ
B��Si����������뵼�����
C������������Һ�������г��õ�������
D������ʳ�ú�������ʳƷ���к����θ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����״̬�£���̬���ӶϿ�1 mol��ѧ�����ʱ��Ϊ���ʡ���֪H—H��H—O��O===O���ļ��ʦ�H�ֱ�Ϊ436 kJ·mol��1��463 kJ·mol��1��495 kJ·mol��1�������Ȼ�ѧ����ʽ��ȷ����
A��H2O(g)===H2(g)��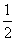 O2(g)����H����485 kJ·mol��1
O2(g)����H����485 kJ·mol��1
B��H2O(g)===H2(g)��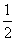 O2(g)����H����485 kJ·mol��1
O2(g)����H����485 kJ·mol��1
C��2H2(g)��O2(g)===2H2O(g)����H����485 kJ·mol��1
D��2H2(g)��O2(g)===2H2O(g)����H����485 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ��ͨ�������з��������������������Ⱦ��
��1����ϡ���ȴ����ܽ�����β���е�CO��NOx��̼�⻯����ת���������ʣ��Ӷ���������β����Ⱦ����֪��
N2(g)+ O2(g)��2NO(g) ��H����180.5 kJ/mol
2C(s)+ O2(g)��2CO(g) ��H��—221.0 kJ/mol
C(s)+ O2(g)��CO2(g) ��H��—393. 5 kJ/mol
д��NO(g)��CO(g)��ת����N2(g)��CO2(g)���Ȼ�ѧ����ʽ ��
��2����NH3��ԭNOx����N2��H2O������NO��NO2�Ļ����3 L������ͬ��ͬѹ��3.5 L��NH3ǡ��ʹ����ȫת��ΪN2����ԭ���������NO��NO2�����ʵ���֮��Ϊ ��
��3���绯ѧ��������ˮ����������Ⱦ�������������£��绯ѧ����NO ��ԭ����ͼ1��AΪ��Դ�� ����������ӦʽΪ ��
��ԭ����ͼ1��AΪ��Դ�� ����������ӦʽΪ ��
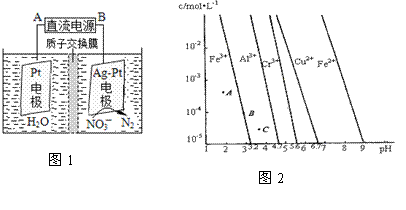

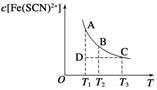
��4��ͨ��������Һ��pH�Թ�ҵ��ˮ�еĽ������ӽ��з��롣ͼ2��ijЩ�������������ڲ�ͬŨ�Ⱥ�pHʱ�ij���——�ܽ�ͼ��ͼ��ֱ���ϵĵ��ʾƽ��״̬������Һ�е�����Ũ��С��1��10-5 mol·L-1ʱ����Ϊ�����ӳ�����ȫ��
����ͬ�����£�Fe (OH)3��Al(OH)3��Cr(OH)3�������ʵ��ܶȻ����������� ��
ͼ��A��B��C�����б�ʾFe(OH)3�ij������ʴ����ܽ����ʵ��� ��
����ͼ�ɵ�Fe(OH)2���ܶȻ���ֵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������йػ�ѧ�����ʾ��ȷ���� �� ��
A���������ױ��Ľṹ��ʽ��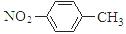
B�Ȼ�淋ĵ���ʽ��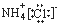
C��������Ϊ16����ԭ�ӣ�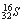
D����Ȳ�����ʽ��CH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ͬ���ʵ���Ũ�ȡ���ͬ�����NaCl��MgCl2��AlCl3��Һ���ֱ���������AgNO3��Һ��Ӧ�����ɳ���������֮��Ϊ________��
��2������������Ũ�ȵ�AgNO3��Һ�У��ֱ������ͬ�����NaCl��MgCl2��AlCl3��Һ��ǡ��ʹ�����е�Cl����ȫת��ΪAgCl��������������Һ�����ʵ���Ũ��֮��Ϊ________��
��3��Na��Mg��Al�ֱ���������������Һ��Ӧ�������������������ͬʱ������Na��Mg��Al�����ʵ���֮��Ϊ________��
��4��������Al�ֱ����Ũ�ȵ����ᡢ����������Һ��Ӧ�������������������ͬʱ��������Һ������������Һ�������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20 mL pH��14����Һ��Ȼ����1 mol/L������ζ���������ɳ�������������������������ϵ��ͼ��ʾ��������˵����ȷ����
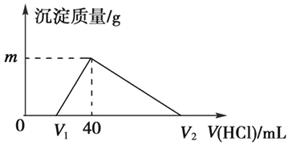
��A��ԭ�Ͻ�����Ϊ 0.92 g�� B���������������Ϊ896 mL(��״����)
��C��ͼ��m��ֵΪ1.56�� D��ͼ��V2��ֵΪ60
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�ֳ���������Һ�У����ܺ����������ӣ�K����Fe3����Ba2����Al3����NH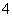 ��Cl����NO
��Cl����NO ��HCO
��HCO ��SO
��SO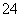 ����������ʵ�飺
����������ʵ�飺
(1)����Һ������ɫʯ����ֽ�ϣ���ֽ�ʺ�ɫ��
(2)ȡ������Һ��������ϡHNO3�ữ��BaCl2��Һ��������ɫ������
(3)��(2)�еij������ˣ�����Һ�м���AgNO3��Һ��������ɫ������
(4)��ȡ��Һ����μ���NaOH��Һ��������ֻ�����к��ɫ�������ɣ��ҳ������������١�
�ɴ˿����ƶϣ�
��Һ�п϶����ڵ�������_________________________________________________��
��Һ�п϶������ڵ�������________________________________________________��
��Һ�в���ȷ���Ƿ���ڵ�������_________________________________________��
SO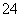 ��Fe3����Ba2����Al3����HCO
��Fe3����Ba2����Al3����HCO ��K����NH
��K����NH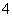 ��Cl����NO
��Cl����NO
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com